Moderna Tumbles After Nonpartisan Pledge, First Sell Rating
Moderna Tumbles After Nonpartisan Pledge, First Sell Rating
(Bloomberg) -- Moderna Inc. fell 13% on Tuesday after regulators and top drugmakers promised the Covid-19 vaccine approval process would be immune from political pressure and the biotech company drew its first sell rating since going public in 2018.
Moderna’s vaccine is one of three leading candidates Wall Street expects may win an emergency use authorization near-term.. But pledges from the Food and Drug Administration and industry leaders have cast a pall over the prospects of a vaccine being approved before the U.S. election.
The public letter signed by nine chief executive officers may not shift study schedules “all that meaningfully,” but it does “help to suggest that the companies will not allow the White House to push vaccines forward that are not ready for public consumption,” Jared Holz a Jefferies health-care trading strategist, wrote in a note to clients.
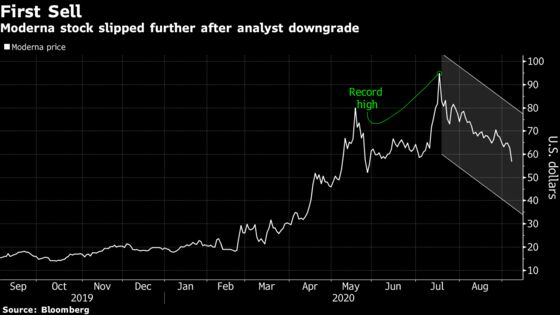
Meanwhile, an SVB Leerink downgrade to underperform from market perform further fueled a selloff in Moderna’s stock. Analyst Mani Foroohar slashed his 12-month price target to $41 from $58 on skepticism the company will be able to keep its early edge in the race for a curative shot and make a profit.
“Loss of mRNA-1273’s lead in clinical development, extension of multiple large volume Covid-19 vaccine contracts to competitors, risk of reduced end-user demand due to increased skepticism for vaccines rushed to market under EUA (emergency use authorization) – make a durable market with oligopoly pricing unlikely,” he wrote of Moderna’s vaccine in his downgrade note.
Moderna’s risk to reward ratio is skewed “sharply to the downside” ahead of a widely anticipated interim phase 3 update and a potential regulatory authorization, Foroohar said. Thirteen analysts tracked by Bloomberg rate Moderna the equivalent of a buy, while two rate the company a hold. Shares are down roughly 40% from a high in July.
Flagging daytrader interest could also be pressuring the stock as phase 3 data comes closer to reality for the companies leading the vaccine race. Aside from Moderna, AstraZeneca Plc and Pfizer Inc. along with German-partner BioNTech SE are expected to have results from large scale studies in thousands of patients before the year is over. “The day of or the day after that data the focus will shift to commercial execution,” Foroohar said in a phone call. “That’s a different skill set of investing as opposed to trading,” where Moderna is positioned particularly poorly, he said.
Another biotech with published early stage vaccine data, Novavax Inc., fell as much as 10.5% before closing down 8.2%. Novavax has surged more than 2,000% this year while Moderna has nearly tripled.
©2020 Bloomberg L.P.