Moderna Rallies on Accelerated Covid-19 Vaccine Push
Moderna Poised for Record on Sped Up Covid-19 Vaccine Push
(Bloomberg) --
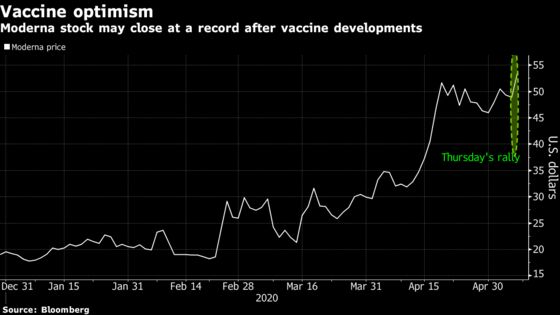
Moderna Inc. jumped as much as 14% on Thursday on accelerated plans to develop an experimental vaccine for Covid-19.
After getting approval from U.S. regulators to proceed on Wednesday, a mid-stage trial is expected to start shortly. The vaccine, mRNA-1273, could be in late-stage study by early summer, Moderna said in a statement. The biotechnology company is preparing for an application for its product to be approved as soon as 2021.
The mid-stage study will enroll 600 healthy adults, with a first look at results expected in the third quarter. Moderna Chief Executive Officer Stéphane Bancel said in a statement that the imminent study start for its vaccine marks a “a crucial step forward.”
Tal Zaks, Moderna’s chief medical officer, on Wednesday discussed plans for a larger vaccine trial this summer that could have results later in the year showing how effective it is. If the vaccine is successful, high-risk people such as those with health conditions or who are elderly could get access sooner, Zaks said in a webcast interview with the publication Stat.
The quickened pace of vaccine development in the U.S. is getting a boost from the Trump administration’s “Operation Warp Speed,” which targets getting a vaccine to most Americans by the end of the year. That ambitious goal could be thwarted by the slower pace of science as well as the challenges of manufacturing massive amounts of as yet unproven inoculations.
Earlier this month, the Cambridge, Massachusetts-based company teamed up with Swiss chemical and and pharmaceutical firm Lonza Group AG to manufacture a billion doses a year of Moderna’s messenger RNA-based vaccine.
“We are accelerating manufacturing scale-up and our partnership with Lonza puts us in a position to make and distribute as many vaccine doses of mRNA-1273 as possible, should it prove to be safe and effective,” Bancel said.
Bancel doesn’t believe “the virus is going away,” and said he hopes many vaccines make their way to the finish line. That broad effort will be necessary to meet global demand, he said. In the U.S., Moderna will look to the government to decide “the allocation that makes sense for the country.”
In terms of ensuring global availability of a coronavirus vaccine, Bancel said “we are going to be constrained for some time.”
Moderna also made changes to its management ranks on Thursday. Lorence Kim, the company’s chief financial officer who was with the company prior to its public listing, plans to depart in August.
Additionally, Jacqueline Miller, formerly of GlaxoSmithKline, will join Moderna to lead infectious-disease development, and Patrick Bergstedt, who recently led global marketing and commercial operations of vaccines at Merck, will join Moderna on June 1 to work on commercializing its vaccines.
©2020 Bloomberg L.P.