Moderna Falls From Record as Euphoria of Vaccine Data Wears Off
Moderna Falls From Record as Euphoria of Vaccine Data Wears Off
(Bloomberg) -- Moderna Inc. shares fell from an all-time high as investors digested early data from a small trial of the company’s coronavirus vaccine, and a $1.3 billion stock sale announced late Monday.
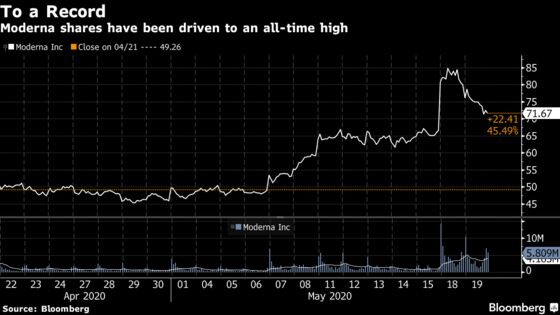
The shares hit a record Monday, and helped raise the broader market, after the Cambridge, Massachusetts-based company released early data from a study of its experimental vaccine. The study was designed to show that the shot was safe, and it was successful on that front. It also contained data from eight patients showing that it produced markers of an immune response, a hopeful sign that it could be effective in fighting the virus in wider tests.
By the standards of medical studies, such early, partial data are considered the territory of specialists and day traders. But after 4.9 million coronavirus infections and more than 320,000 deaths around the globe, a glimmer of medical hope -- however thin -- has been enough to shift trillions of dollars in value in the markets.
After Monday’s 20% rise to $80, the company announced after the close that it would sell 17.6 million shares at $76. On Tuesday, Moderna closed down 10% to $71.67. A gradual slide in the shares that began when trading opened gained momentum after a report from the health publication Stat highlighted the early nature of the vaccine data, helping to drag down the broader market.
Stat cited the lack of a press release from the U.S. National Institutes of Allergy and Infectious Diseases, which partnered with Moderna on the trial. The publication also cited experts who said they were waiting to see more data from the company before drawing a conclusion.
One Wall Street analyst raised the core doubt about the experimental vaccine, called mRNA-1273, while raising his price target on the stock from $80 to $112 a share.
“We still do not know whether mRNA-1273 is in fact protective against coronavirus infection in humans,” said BMO Capital Markets’ George Farmer. Translation: Nobody knows if the vaccine works yet. The company has also not said how it plans to make money on the vaccine.
The same market-shifting dynamic happened a few weeks before with Gilead Sciences Inc.
In April, the company and the broader market surged after a report that the company’s Covid-19 drug had helped a small group of patients in Chicago. A week later, they plunged -- and sent the S&P 500 down as well -- after another report that the drug might not work, after all.
The drug, remdesivir, has since been cleared by the U.S. Food and Drug Administration under an emergency authorization while more data are awaited.
Speculation in shares of companies developing therapies for Covid-19 has gotten so rampant that it doesn’t take much news to cause dramatic moves in stock prices. Aldeyra Therapeutics Inc., a biotech firm with a market value of just over $100 million, surged as much as 19% in late trading Tuesday after the company scheduled a call to “provide a Covid-19 development update.”
Moderna Data
A vaccine is considered a crucial step toward lifting social-distancing measures and safely reopening economies, schools and events around the globe. The pandemic has spurred a global race by drugmakers, academic institutions and governments to find a vaccine.
In the phase 1 test of Moderna’s vaccine, the researchers looked at blood samples from the test subjects and whether the vaccine helped them generate antibodies that could theoretically fight off an infection. The researchers found that at two lower dose levels used in the study, levels of antibodies found after getting a second booster shot of the vaccine either equaled or exceeded the levels of antibodies found in patients who had recovered from the virus.
In 25 people who got either of the two smaller doses used in the study, researchers reported that the levels of antibodies equaled or exceeded the levels of antibodies found in patients who had recovered from the virus.
A second analysis, evaluating the quality of those antibodies, was only available for eight of the people because it takes longer to perform. But in all eight people, the vaccine successfully stimulated the body to create antibodies capable of neutralizing the virus in the test tube, so it can no longer infect cells.
©2020 Bloomberg L.P.