Moderna Extends Rally With Another Vaccine in the Works
Moderna Extends Rally With Another Vaccine in the Works
(Bloomberg) -- On the heels of its biggest one-day gain Tuesday, Moderna Inc. touched an intraday record after revealing the development of an experimental vaccine for a virus tied to birth defects is advancing faster than expected.
That’s the second piece of good news for the biotech this week, which surged 28% yesterday after revealing that vials of a vaccine for the coronavirus had been shipped to the National Institutes of Health’s National Institute of Allergy and Infectious Diseases for clinical testing.
Enrollment in mid-stage study of Moderna’s mRNA-1647, a vaccine for cytomegalovirus or CMV, an infection that can lead to birth defects, has been completed for two of the three dosing arms. A third arm is well on its way to being completed, with more than 85% of the needed patients already enrolled.
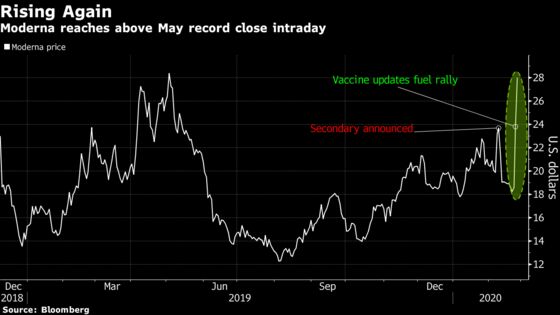
Moderna expects to have a CMV vaccine in the final stage of testing next year, the company said in its quarterly earnings report. That could bring the Cambridge, Mass.-based company’s medicines that use messenger RNA to alter stem cells and treat diseases one step closer to reality.
On the company’s fourth quarter call, management declined to give analysts any additional information on plans to move forward with the coronavirus vaccine or how profitable it might be, instead deferring questions to the NIH. NIAID Director Anthony Fauci told reporters during a briefing yesterday that he predicted a human vaccine trial for the coronavirus would start in less than two months, while it might take a year and half for a vaccine to reach the public.
The stock rose as much as 26% on Wednesday, briefly reaching an intraday record of $29.98.
--With assistance from Michelle Fay Cortez.
To contact the reporter on this story: Cristin Flanagan in New York at cflanagan1@bloomberg.net
To contact the editors responsible for this story: Catherine Larkin at clarkin4@bloomberg.net, Janet Freund
©2020 Bloomberg L.P.