Millions of Applications Could Hit FDA as E-Cig Deadline Arrives
Millions of Applications Could Hit FDA as E-Cig Deadline Arrives
(Bloomberg) -- The U.S. Food and Drug Administration is girding for a possible deluge of tens of millions of e-cigarette review applications by a Wednesday deadline, setting up a process to prioritize prominent brands like Juul and preparing a labor-intensive effort to root out companies making illegal sales.
Manufacturers who want to continue selling e-cigarettes must submit an application for FDA authorization by Wednesday. The deadline is a climactic moment for the e-cigarette business, which has grown into a more than $5 billion industry while drawing scrutiny for soaring rates of teen use.
The application process is likely to swamp regulators with work. E-cigarette makers must submit each individual product for review -- and many sell numerous versions with different levels of nicotine or different flavors.
The review will likely eliminate some brands while freeing others from years of uncertainty. Products that aren’t pursuing clearance from the FDA must now come off shelves. Those that are seeking the blessing of regulators can remain on the market for as long as one year while the agency evaluates them.
More than 400 million e-cigarettes and other newly regulated tobacco products are eligible for review, though it isn’t clear how many will apply, Mitch Zeller, the director of the FDA’s Center for Tobacco Products, said in an interview. Anticipating a rush of last-minute applications, the FDA is developing a strategy to choose which to review first.
The FDA plans to prioritize the most popular products while also creating a system to pick submissions from smaller companies to review, Zeller said. They also plan to evaluate entirely new items that aren’t subject to the deadline and must receive the agency’s permission before hitting shelves. The final approach will depend on how many submissions the agency receives.
Market leader Juul Labs Inc. and a number of other large companies have already filed applications.
Covid Complications
A number of smaller companies have asked the FDA to again extend the deadline, which the agency pushed to September from May because of the Covid-19 pandemic. The FDA denied those requests and is instead telling manufacturers to explain in their applications how the public-health crisis prevented them from completing all the required scientific requirements, Zeller said.
To help enforce the deadline, the FDA plans to publish a list of the products pursuing review that retailers can reference. When the document will be available will depend on how many applications the agency receives and will require staff to track down additional information from companies to comply with the complex law. In the meantime, Zeller said stores should check with distributors and manufacturers.
The agency plans to send warning letters and use other enforcement tools as needed, such as blocking specific products from being imported into the U.S., Zeller said.
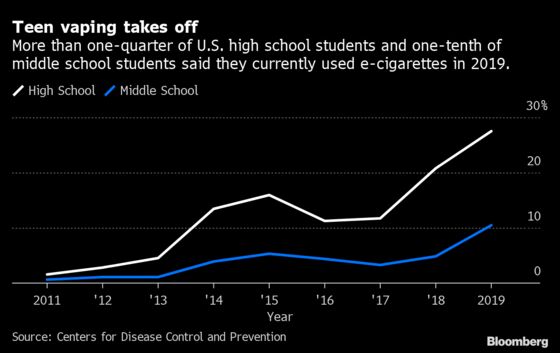
The FDA will evaluate which e-cigarettes are appropriate for the protection of public health, a standard that requires proving products can benefit adult smokers without attracting youth or nonsmokers. Skyrocketing rates of teen use have overshadowed any possible benefits of e-cigarettes.
“Absolutely, unequivocally, youth use is the biggest issue for people thinking about tobacco,” said Stefanie Miller, a regulatory risk analyst and managing director at FiscalNote Markets.
All eyes will be on Juul, the company that President Donald Trump’s first FDA commissioner, Scott Gottlieb, has blamed for the teen vaping epidemic. Juul, which has said it never targeted underage users in its marketing, has curtailed its sales of flavored products and pulled back from many markets as it focuses on gaining U.S. clearance for its remaining products.
Juul filed applications in July for its device, tobacco and menthol-flavored pods in 3% and 5% strengths. The FDA is now reviewing them, the company has said.
Juul didn’t seek permission to reintroduce the sweet flavors like mango and mint that a federal survey identified as the most popular among students last year. Closely held Juul’s largest investor is Marlboro maker Altria Group Inc., which owns a 35% stake in the e-cigarette company.
Political Forces
Regulators banned flavors from some of the most popular e-cigarettes and Congress raised the federal smoking age to 21 earlier this year in hopes of stemming youth use. Public-health advocates are urging the FDA to sweep the market of all flavored e-cigarettes through the review process. Some companies, including Reynolds American Inc. and NJOY Inc., have applied to reintroduce fruity flavors.
The Trump administration last fall backed away from its plan to prohibit all flavors, including menthol, from every type of e-cigarette after intense outcry.
In terms of the review, Zeller said the agency’s job is to follow the science.
“I have complete confidence in the system that we set up,” Zeller said. “These are decisions that will be made by the right people and based upon the best available evidence.”
Michael R. Bloomberg, the majority owner of Bloomberg LP, the parent company of Bloomberg News, has funded efforts to ban flavored vaping products.
©2020 Bloomberg L.P.