Goldman Called 2019 ‘The Year of NASH,’ Others Say Not So Fast
Goldman Called 2019 ‘The Year of NASH,’ Others Say Not So Fast
(Bloomberg) -- The fatty liver disease that some on Wall Street view as health care’s next blockbuster market may take another year or two to develop, according to analysts.
While firms like Goldman Sachs have hailed 2019 as “the year of NASH” with late-stage data expected from Gilead Sciences Inc. and Intercept Pharmaceuticals Inc., other analysts say the first study results will only scratch the surface. Next year’s results will be for individual drugs, not the combination therapies that doctors and analysts think will be key to treating the different ways the disease can manifest itself in patients.
“There’s part of the market that thinks 2019 is the year for NASH. I don’t know if that’s entirely true,” Mizuho analyst Salim Syed said in an interview. He expects that real breakthroughs may have to wait until combo data in 2020 or 2021.
NASH, or nonalcoholic steatohepatitis, is a significant form of chronic liver disease that affects both adults and children and can require a liver transplant. Much of the debate on treatment centers on which drug targets will work for different aspects of the disease, how to easily diagnose patients and how drugs that work differently can be used in tandem. And that’s before health insurance companies even consider covering the costs of these treatments.
The first look at late-stage results should come from Gilead’s selonsertib programs and Intercept’s study of Ocaliva in the first half of 2019. The presentations should offer guidance on what drugs may best work in combination and will set a bar for medicines in earlier stages of development, according to Piper Jaffray analyst Tyler Van Buren.
“The long-term goal is to combine some of these mechanisms to get both reduction in liver fat, fibrosis and metabolic parameters, but those pivotal studies are further out,” Van Buren said by telephone, noting that some companies have more difficult targets in their larger late-stage studies. “2019 is going to be all about fibrosis as multiple Phase 3 trials with fibrosis-related primary endpoints are expected to report throughout the year.”
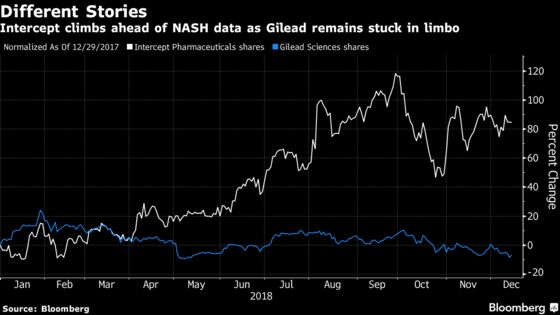
Some of the Nasdaq Biotechnology Index’s top performers this year are NASH names as investors swoon over a market that some estimate could surpass $20 billion. Intercept shares are up more than 85 percent in 2018, while Viking Therapeutics Inc. has more than doubled. Other gainers include Enanta Pharmaceuticals Inc., which has risen more than 25 percent, and Madrigal Pharmaceuticals Inc., which is up 24 percent.
Gilead has fallen 7 percent this year as large-cap biopharmaceutical stocks failed to excite investors like their small-cap brethren as investors seek innovation.
“We are nearing ‘the end of the beginning’ of drug development in NASH with an approval in the near future most likely, but it will be a long road with new drugs, biomarkers, combinations and trial designs,” said Scott Friedman, a liver disease expert who consults for a number of NASH-focused companies and researchers.
To contact the reporter on this story: Bailey Lipschultz in New York at blipschultz@bloomberg.net
To contact the editors responsible for this story: Catherine Larkin at clarkin4@bloomberg.net, Cristin Flanagan, Steven Fromm
©2018 Bloomberg L.P.