Intercept Drops as FDA Refuses Speedy Approval of Liver Drug
Intercept Drops as FDA Refuses Speedy Approval of Liver Drug
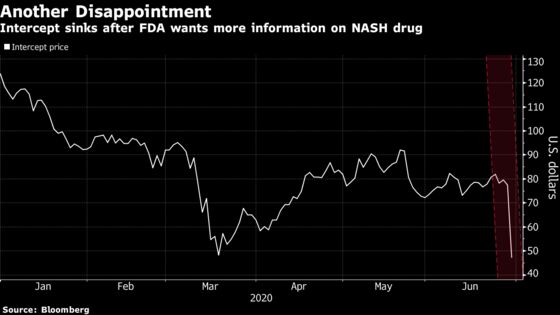
(Bloomberg) -- Intercept Pharmaceuticals Inc. shares plummeted Monday as U.S. regulators dashed hopes for an accelerated approval of what investors thought would be the first medicine to treat tens of millions of people with a deadly liver disease.
The New York-based company sank as much as 41%, the biggest drop ever for the stock, as the U.S. Food and Drug Administration further delayed its bid for a treatment for a fatty liver disorder known as nonalcoholic steatohepatitis, or NASH. The FDA suggested in its so-called complete response letter that the once-promising candidate’s benefit isn’t clear and doesn’t outweigh the potential harm, leaving its future uncertain.
“Today represents a setback,” Chief Executive Officer Mark Pruzanski said on conference call. “We very much disagree with the assessment provided in the CRL related to the benefit-risk profile,” he said, calling the decision “premature” and based on an “incomplete review.”
The FDA didn’t immediately respond to calls and emails seeking comment.
NASH, which afflicts tens of millions of Americans, is marked by a build-up of fat and scarring in the liver. In the mildest cases, patients can be fairly asymptomatic, while the disease can progress to deadly liver cancer in others. Intercept’s drug, obeticholic acid, known as OCA, has been shown to improve the scarring that occurs when the organ’s tissue is repeatedly damaged or inflamed.
Postponed Meeting
Some on Wall Street had predicted that the FDA might not give OCA an early nod for treating NASH after an advisory committee meeting with the drugmaker was delayed in May.
Pruzanski, who founded the company from his New York City apartment nearly two decades ago, said the FDA hasn’t been communicative about its shifting expectations for data or its timeline for reviewing it. He called it “inexplicable” that the FDA would release a decision before meeting with stakeholders.
Intercept intends to request the agency reschedule the meeting “considering that the public health issue that NASH represents as far as I can tell is only one of two chronic diseases out there that has no treatment,” Pruzanski said. The other is Alzheimer’s disease, he said.
The big question for Intercept will be if another interim look at the study known as Regenerate already in progress would win regulatory approval or if the FDA will insist on data on liver-related outcomes first, Stifel analyst Derek Archila wrote in a client note. Those results aren’t expected until late 2022, he said.
This isn’t the first time OCA has run into issues with U.S. regulators. In 2017, the FDA issued a warning after patients with a rare liver disease died while getting the drug. The medicine, which is already approved in a bile-duct disorder, later had a boxed warning about potential overdoses added to the label.
Large pharmaceutical companies like Pfizer Inc., Bristol-Myers Squibb Co. and Eli Lilly & Co. and a smattering of biotechs have lagged behind Intercept in the race to treat a growing population of people sick with the disease, which is prevalent among those with weight-related conditions like diabetes and obesity. One rival, Madrigal Pharmaceuticals Inc., is expected to have late stage results in the latter half of 2021. Some smaller firms like Viking Therapeutics Inc. and Inventiva are at earlier stages of development.
Intercept shares have faced volatility over the last year as investors expressed their disappointment that the biotech hadn’t been acquired by a bigger drugmaker based on its late-stage data. More broadly, Wall Street’s appetite for NASH drugs waned in the wake of numerous failed trials out of Gilead Sciences Inc., Genfit Inc. and others.
Investors are reading this as negative for the NASH space overall, but with other therapies showing bigger benefits, “we’d disagree,” Archila said in a phone call. Madrigal’s treatment could “leap-frog past Intercept,” to be the first NASH drug to make it to the market, he said.
For now, the FDA’s decision on Intercept’s drug is hurting those companies following it in the dash to the finish line. Madrigal fell as much as 11%, Viking slipped 3.8% while yet another competitor NGM Biopharmaceuticals dropped 13%.
©2020 Bloomberg L.P.