Indivior Shares Plunge After Suboxone Patent Fight Setback
Shares of Dr. Reddy’s Labs has gained nearly 4.6 percent, the most since over two months, to Rs 124.65 apiece.

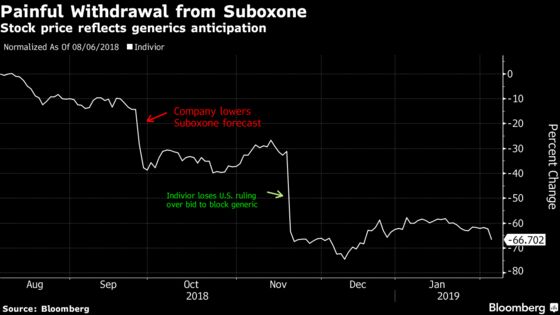
(Bloomberg) -- Indivior Plc shares plunged as much as 25 percent after a U.S. court ruling that may allow Dr. Reddy’s Laboratories Ltd. and others to start selling generic versions of Suboxone film, the company’s treatment for opioid addiction.
Dr. Reddy’s and Alvogen Pine Brook LLC will likely start selling cheaper versions of Suboxone film as soon as a sales ban lapses on Feb. 11, potentially wresting as much as 80 percent of the market share from Indivior’s branded product within months, the Slough, England-based company said in a statement. Mylan NV may also launch a generic soon, analysts said.
Indivior’s drug has been widely used in helping patients kick powerful opioid painkillers that are at the center of a deadly U.S. epidemic of overuse and abuse. The company has lost more than half its value since a Nov. 20 ruling that threatened its ability to maintain exclusivity for its biggest product. The shares as low as 84 pence Tuesday in London.
The company is working to promote a new opioid addiction treatment called Sublocade, “which has now become more critical to the business,” said James Vane-Tempest, an analyst at Jefferies LLC in London. Indivior may issue guidance that’s lower than analysts’ consensus when it reports earnings on Feb. 14, he wrote in a note to clients.
The U.S. Court of Appeals for the Federal Circuit on Monday denied Indivior’s request to reconsider its Nov. 20 ruling that a trial judge was wrong to block Dr. Reddy’s while a patent-infringement suit is pending. The sales ban had remained in force, however, to give Indivior time to challenge the Federal Circuit’s 2-1 decision.
The mandate directing the lower court to lift the ban will be issued on Feb. 11, the appeals court said. Indivior plans to file an emergency motion to keep the mandate in place.
Suboxone Film, first approved by the U.S. Food and Drug Administration in 2010, is considered faster and easier to take for some patients than tablets. The tablet form of Suboxone was first approved by regulators in 2002 and is already available as a generic.
Almost 875,000 people in the U.S. received Suboxone last year, according to Indivior. The U.K.-based drugmaker reported $768 million in sales during the first nine months of the year, with the bulk coming from Suboxone.
--With assistance from Lisa Pham.
To contact the reporters on this story: Susan Decker in Washington at sdecker1@bloomberg.net;Marthe Fourcade in Paris at mfourcade@bloomberg.net
To contact the editors responsible for this story: Eric Pfanner at epfanner1@bloomberg.net, John Lauerman, Thomas Mulier
©2019 Bloomberg L.P.