Gilead’s Remdesivir Can Hardly Live Up to the Hype
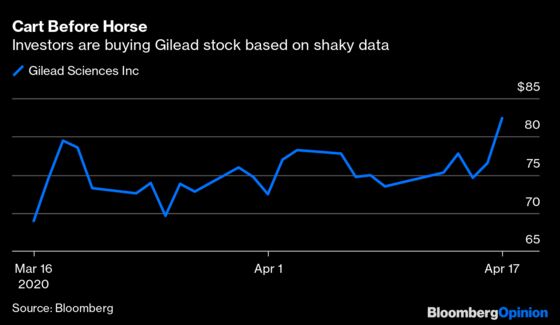
(Bloomberg Opinion) -- The latest round of hype on Covid-19 drugs began Thursday afternoon when Stat News reported on a leaked video discussion about Gilead Sciences Inc’s remdesivir. A Chicago doctor who had tested it on severely ill patients suggested it was working — that most of those who were given the medicine recovered and were discharged. The market is reacting as if the drug were already a commercial hit: Gilead shares have been up as much as 12% in early trading.
Investors should be clear about what this report is and isn’t, however. It is a promising anecdote that suggests the drug might be useful. It is also extremely limited information from a small portion of a trial taken out of context. In other words, it’s miles from providing proof that remdesivir cures Covid-19. The story is even further from being a reasonable basis for a multibillion-dollar stock move.
Anecdotal data gets people excited, especially when it sends a message they want to hear. But it’s not especially good at predicting whether a drug will work. Just a few weeks ago, similar anecdotal reports suggested that hydroxychloroquine, an older malaria drug, could be a Covid-19 miracle medicine. More robust trials show side effects and limited efficacy.
Before this video emerged, the data on remdesivir was mixed. A New England Journal of Medicine report on compassionate use of the medicine also suggested promise, but it was likewise anecdotal. On the other hand, Chinese researchers running extensive controlled studies on it recently stopped them. A slowing outbreak hurt enrollment. However, RW Baird & Co. analyst Brian Skorney argues that if the drug looked as effective as the Stat report suggests, those researchers would “certainly” have reported data.
The Stat report has sparked particular exuberance because it highlights significant effects in very sick patients — evoking images of people being brought back from death’s door. But in selecting severely ill patients, this trial screened out those requiring mechanical ventilation and those with multi-organ failure or kidney or liver impairment, according to Skorney. That leaves a group of patients who probably had a better chance of recovery from the start, with or without remdesivir.
The Chicago hospital is just one site in a larger Gilead-sponsored study that lacks a control arm. Even when all the data are in, it will be difficult to know how well these patients might have done with supportive care or other therapies. The world may need to wait longer for a more conclusive verdict from a double-blinded placebo-controlled clinical trial that’s being run by the National Institute of Allergy and Infectious Diseases.
Even if the NIAID study succeeds, or the FDA approves the medicine based on earlier data, remdesivir isn’t certain to become a financial or medical blockbuster. The drug may work only relatively early in the course of a coronavirus infection. And because doctors must infuse it over several days, its uses are somewhat limited. The manufacturing process is also long and complicated, and political pressure will keep Gilead from pricing aggressively.
Absent superlative real data or a manufacturing breakthrough, it’s hard to imagine sales of the medicine justifying the addition of billions to Gilead’s market cap. By fall, we’ll have data from immunosuppressive drugs that have generated promising anecdotal data of their own and from novel antibodies that may supplant remdesivir.
The best hope for Gilead’s drug is that it will become part of the toolkit for fighting Covid-19. Realizing that promise will take much more than a video.
This column does not necessarily reflect the opinion of Bloomberg LP and its owners.
Max Nisen is a Bloomberg Opinion columnist covering biotech, pharma and health care. He previously wrote about management and corporate strategy for Quartz and Business Insider.
©2020 Bloomberg L.P.