How a Tainted Heart Drug Made in China Slipped Past the FDA
How a Tainted Heart Drug Made in China Slipped Past the FDA
(Bloomberg) -- U.S. regulators sent a stark warning to American consumers last year: A Chinese-made heart drug taken by millions of people was contaminated with a possible cancer-causing chemical.
The Food and Drug Administration oversaw a recall of the tainted pills. But even as it did so, the agency that helps safeguard a global supply chain of drugs was conducting fewer inspections of pharmaceutical plants in the country where the problem originated.
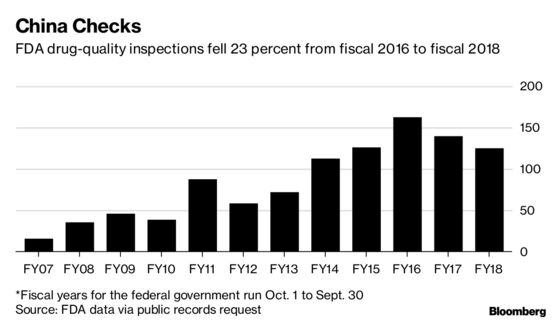
Treatments made by Chinese companies now account for almost one of every 10 generic drugs cleared by the FDA for sale. But agency inspections meant to ensure that approved drugs are meeting U.S. standards fell almost 11 percent, to 125, in China for the fiscal year that ended Sept. 30, compared with the previous year, according to data obtained by Bloomberg through public-records requests.
Using hundreds of pages of the U.S. government documents, Bloomberg has spent the last year reporting on a supply chain that reaches around the world and ends inside American medicine cabinets. While overall inspections of that network are down, records show that those that do get done—from West Virginia to China and India—raise doubts about the data meant to prove drugs are safe and effective.
Those doubts are sometimes overruled by FDA management. At the plant that set off the heart-drug recall in July of last year, an FDA inspector had determined in May 2017 that some of the drugs it was supplying to the American market might be substandard, according to an inspection report obtained by Bloomberg.

The FDA inspector’s 2017 visit had turned up a number of problems. The Chinese drugmaker, Zhejiang Huahai Pharmaceutical Co. Ltd., had omitted from official records quality-test results that showed unnamed drugs failed to meet U.S. standards, and instead recorded passing grades, the FDA inspector wrote in his report. The inspector recommended that the agency send Zhejiang Huahai, one of China’s largest exporters of pharmaceuticals, a warning letter that likely would have meant it couldn’t gain approval to make any new generic drugs in that factory until it cleared up the list of problems.
But four months later, FDA managers at the agency’s Silver Spring, Maryland, headquarters overruled the inspector. Zhejiang Huahai Pharmaceutical was allowed to avoid those penalties and address the problems itself—possibly missing a chance to detect the cancer-causing contaminant more than a year earlier than it was.
“That’s not OK to just wave it off,” Kevin Schug, an analytical chemistry professor at the University of Texas, Arlington, said after reading the inspection report at the request of Bloomberg. Schug specializes in the type of testing that drug companies use to conduct quality checks. “I certainly would not want to take [any] drug had it gone through that process.”
An FDA spokeswoman, Sarah Peddicord, said patient safety dictates the agency’s response. After the 2017 inspection uncovered the problem with impurities, “FDA reviewed the inspectional findings and considered the company’s proposed corrective actions when deciding what action to take.”
More and more medications that Americans consume are coming from China, where production costs are lower. Chinese-made drugs accounted for 8 percent of FDA generic approvals in 2018 through Oct. 30, up from 1 percent in 2015, according to Morris Borenstein, a senior Moody’s analyst.
Yet inspections of generic makers in China have fallen. The recent decline pushed the number of FDA inspections in the country below what they were a few years ago, when the agency vowed to beef up its overseas capability. President Donald Trump’s FDA commissioner, Scott Gottlieb, has made ushering more generic drugs to market a priority as part of the president’s effort to lower prices.

In defending his agency’s approach, Gottlieb said it uses a formula that weighs risk factors, including when a factory was last inspected and what the inspection found—not geography.
“If you’re thinking about this from a risk standpoint—from the people that do the intelligence work that know where the risks are—it's not just that we've done x number of inspections over x number of years of x number of facilities,” Gottlieb said in an interview. “The points of risk change as the nature of the supply chain has changed.”
So-called surveillance inspections done in fiscal 2017, which included Gottlieb’s first few months in office, totaled 140 in China, a 14 percent decrease from the 163 the FDA did in fiscal 2016. The agency uses surveillance inspections to ensure companies are complying with U.S. manufacturing standards and to verify the accuracy of companies’ internal quality tests.
In an update on the heart-drug recall Friday, the FDA said any additional inspections should focus not on a particular country, but on facilities that make the treatment, which goes by the name valsartan. “We remain confident in the use of FDA’s current risk-based approach, which does not include geographic location as a risk factor, and in our continuing ability to protect patients from products that have the potential to cause harm,” the agency said.
Zhejiang Huahai didn’t respond to questions about whether it could have found the potential carcinogen, a chemical called NDMA, sooner. Jun Du, chief executive officer of Zhejiang Huahai subsidiary Prinston Pharmaceutical Inc., said in an email that the FDA closed its 2017 inspection of the Chinese plant after the company submitted detailed responses to the inspector’s observations.
“At all times from the inception of the recall, we have been in close communication with the FDA, and have collaborated throughout with agency staff, our customers and patients,” Du said.
Zhejiang Huahai was supplying the active-ingredient valsartan—a widely used treatment for high blood pressure that is taken alone, as well as sold in combination with other cardiovascular drugs—for major companies such as Teva Pharmaceutical Industries Ltd., the largest generic drugmaker in the world. After the FDA flagged the contamination problem, Teva in July started recalling valsartan and valsartan combined with other drugs. Since then, other companies have recalled it as well, including some that didn’t purchase valsartan from Zhejiang Huahai, such as Mylan NV, the second-largest generic-drug company, for containing a similar cancer-causing ingredient, NDEA.
Zhejiang Huahai has had problems with its data before. In 2016, Chinese regulators asked drug makers to withdraw any applications to sell new drugs that may have contained false or incomplete data. Zhejiang Huahai pulled its applications for generics for epilepsy, blood pressure and depression, saying at the time that the issues related to flawed testing by a local Chinese contract research organization, affiliated with a major hospital in China.
The increasing number of companies buying their ingredients from China raises concerns that such problems are more widespread, said Randall Zusman, director of the hypertension division at Massachusetts General Hospital Heart Center.
“I found it surprising that Teva, which has their own manufacturing plants, was buying the Chinese valsartan for their products,” Zusman said. “They found it economically appropriate to buy from this Chinese supplier, which shows just how widespread this could become if that continues to be the case.”
Zusman said one of his colleagues consulted him about a patient who developed bladder cancer while taking valsartan. It’s unclear if the valsartan contributed to the cancer, Zusman said, but it's a concern. “Obviously, it’s very scary to the patient,” he said. “And it’s disillusioning to me to think that I might have prescribed a drug that could have caused a malignancy.”
He is currently not starting any of his patients on valsartan. While there are alternatives, the recall has raised doubts in his mind about the drug.
“I am rethinking every time I’m prescribing one of these,” Zusman said of valsartan products.
The FDA inspector who visited Zhejiang Huahai’s factory in the city of Linhai wrote in his inspection report that the company has a practice of recording passing scores for drugs that originally fell short of U.S. standards on routine quality tests. He said his findings cast “a cloud of uncertainty over the accuracy of test results” that are used to gain clearance to sell drugs in the U.S., according to documents from the public-records request.
The quality tests, which are standard practice in pharmaceutical manufacturing, check for the amount of active ingredient in a drug and scan for potential contamination. The FDA inspector recommended the company be given a warning letter, one of the FDA’s strongest rebukes: It can set in motion restrictions on whether a company can sell products in the U.S. Companies that get warning letters also often have to undergo rigorous evaluation of their testing methods.
According to a memo obtained through the public-records requests, the FDA managers who overrode the inspector’s concerns about Zhejiang Huahai decided to let the company correct its problems, choosing not to place any restrictions on the drugmaker. The FDA managers said that the agency had found no violations during inspections in 2010 and 2014, and that the test results in question hadn’t affected the final products.

About a year after the inspector’s concerns were overruled, a company that buys valsartan from Zhejiang Huahai spotted an impurity in the Chinese company’s active ingredient that was discovered to be the potentially cancer-causing chemical NDMA. Zhejiang Huahai’s plant that makes valsartan was then banned from sending drugs to the U.S. (The FDA didn’t identify the customer that discovered the impurity.)
The FDA inspector who originally visited the factory had written in his report that the failed quality tests the company had omitted from official records included ones that flagged impurities in unnamed drugs that the company didn’t attempt to identify.
Zhejiang Huahai was supposed to figure out how to identify the impurities or get rid of them, said Schug, the analytical chemistry professor. If NDMA was being introduced or created during the manufacturing process, testing would likely identify it, he said.
“They certainly should have caught it, and they should have modified the procedure to correct it,” Schug said.
Zhejiang Huahai didn’t comment on specific questions about the failed testing. Inspectors revisited the Linhai factory in July and August, after the valsartan recall started. The FDA again found Zhejiang Huahai had omitted tests that flagged unknown impurities, according to a warning letter the agency sent the company Nov. 29.
The letter stated that if Zhejiang Huahai “had investigated further,” workers may have found indicators “alerting you to the presence of NDMA.” The FDA also said in the letter that it had “grave concerns” about the presence of potentially cancer-causing impurities in all pharmaceutical ingredients manufactured by Zhejiang Huahai, not just valsartan.
Zhejiang Huahai has cooperated with the FDA throughout its investigation, said Du, the Prinston chief executive officer. “In detailed analyses and corrective action plans, the company has been and is currently addressing each of the FDA’s 2018 observations, as well as items noted by the FDA more recently in its warning letter,” he said.

Since the revelation that Zhejiang Huahai’s valsartan contained NDMA, the FDA has repeatedly updated the recall notice as new samples of valsartan—and even similar drugs called losartan and irbesartan—are found to be contaminated. The agency is attempting to determine how the chemical made its way into the products and is reviewing the companies’ manufacturing processes to determine if they might risk forming NDMA or NDEA.
Not all the companies that have recalled the drug received it from Zhejiang Huahai, but manufacturers of the recalled valsartan products used a similar process to make the pills. That process is different from the brand-name version of valsartan called Diovan, made by Swiss drugmaker Novartis AG. Diovan hasn’t been found to contain NDMA or NDEA and hasn’t been recalled.
Zhejiang Huahai’s valsartan contained “significantly higher” levels of NDMA than other recalled versions of the drug, the FDA said in its November warning letter.
U.S. regulators expect companies to police themselves. “The FDA’s review of records relies on manufacturers conducting appropriate tests that are capable of detecting impurities,” said Peddicord, the agency spokeswoman. “It is the manufacturer’s responsibility to ensure these tests are based on adequate assessments of what impurities are expected to develop during the manufacturing process.”
The agency said it has been working with valsartan makers affected by the recall “to minimize or eliminate” the risk of forming probable cancer-causing impurities.
In the case of Zhejiang Huahai, the company had last made changes to its processes in 2011, according to the FDA. And the agency said the potential carcinogen may have been in the valsartan for as long as four years.
To contact the editor responsible for this story: Flynn McRoberts at fmcroberts1@bloomberg.net, Timothy Annett
©2019 Bloomberg L.P.