Bristol Myers Deal-Linked Derivative Surges on Inspection Hopes
Bristol Myers CVR Spikes as Traders Position Into Key Deadline
(Bloomberg) -- The deal sweetener Bristol Myers Squibb Co. offered as a part of its Celgene Corp. purchase spiked as traders positioned ahead of a key year-end deadline and shared optimism related to a needed regulatory inspection.
The $9 all-or-nothing payment jumped as much as 55% to the highest level in about three weeks as investors weighed whether U.S. regulators could inspect a facility key to the derivative’s payout in time for a year-end deadline.
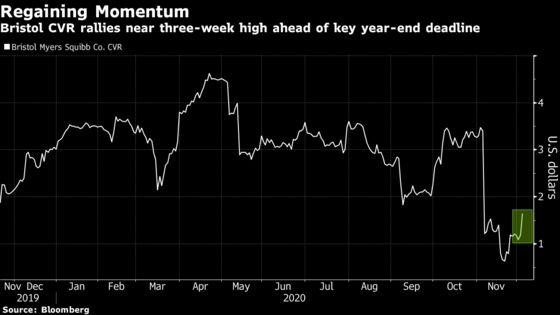
While traders await word from the company on the state of the inspection, they shared commentary from another drug manufacturer at an Evercore ISI event Wednesday that said a site inspection had just been completed. Fans of the so-called contingent value right, or CVR, viewed the update as a positive sign that the Food and Drug Administration is still inspecting facilities amid the pandemic.
The derivative, which has been volatile since plummeting a month ago, depends on FDA approval of the company’s cancer drug lisocabtagene maraleucel by the end of the year as well as approval of a second drug, idecabtagene vicleucel in multiple myeloma, by March 31.
Read more: Bristol CVR Surges as FDA Head’s Tweet Sparks Investor Optimism
Investors have been doing their best to read the tea leaves for what would prove to be a very profitable trade if Bristol Myers can clear the two remaining hurdles. The derivative spiked last week as traders circulated a tweet from FDA Commissioner Stephen Hahn, which signaled the agency is ramping up inspections of drug manufacturing sites.
©2020 Bloomberg L.P.