Bristol-Myers CVR Sinks After FDA Rejects Key Cancer Drug Filing
Bristol-Myers CVR Sinks After FDA Rejects Key Cancer Drug Filing
(Bloomberg) -- Bristol-Myers Squibb Co.’s announcement that U.S. regulators rejected their filing for its Bluebird Bio Inc.-partnered cancer treatment bb2121 raised cause for concern among investors as a deal sweetener for its purchase of Celgene Corp. sank.
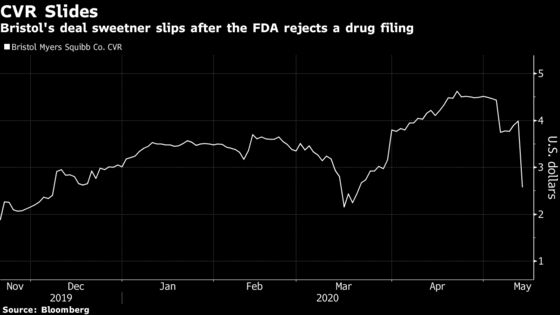
The Refusal to File letter from the Food and Drug Administration deals a blow to an all-or-nothing $9 per share deal payment and casts more doubt on Bristol-Myers’s ability to meet the three requirements for the payout. The contingent value right, or CVR, crashed as much as 43% in early trading Wednesday, the worst drop since it first started trading in November.
The filing rejection was based on issues tied to manufacturing and was not related to any safety of efficacy data, the companies said in a joint press release. There is no need for new clinical studies, and the pair expect to resubmit the filing for bb2121 -- also known as idecabtagene vicleucel or ide-cel -- no later than the end of July, meaning approval by the March 31 deadline is still on the table.
While a potential eight-month review pending a priority review designation will keep the deadline intact, Mizuho analyst Salim Syed weighed if two-and-a-half months is enough time for the duo to fix the manufacturing issue and resubmit the filing. “Bristol Myers seemed quite adamant on the call this morning that it will be able to resubmit by end of July,” he wrote in a note to clients. Then after a follow-up conversation with management, Syed said a July deadline “should be doable.”
In order to receive the full value of the CVR, investors also need Bristol-Myers to gain FDA approval of another former Celgene drug, lisocabtagene maraleucel, by the end of the year. A regulatory update last week for the drug known as liso-cel spooked some investors after the FDA extended a review for the cancer drug by three months. If neither of the drugs make it, the CVR is worthless.
For those tracking the CVR’s progress, the company cleared its first hurdle with the March approval of Zeposia. Bristol-Myers shares were little changed in early trading Wednesday. Bloomberg Intelligence analysts Sam Fazeli and Cinney Zhang wrote that the rejection is a problem for CVR holders “rather than an issue for Bristol,” as the drug is expected to account for just 1% of 2023 sales.
Bluebird analysts were more optimistic at first blush with shares little changed on Wednesday. While the announcement is “surprising” and “frustrating,” Stifel analyst Benjamin Burnett wrote that the bank still sees a 95% chance of the drug winning approval. However, because Bluebird isn’t a part of the CVR and its milestones, Burnett’s research note didn’t touch on the impact for holders of the derivative.
©2020 Bloomberg L.P.