Bristol Myers CVR Dealt Blow as Key Drug Faces Approval Hurdle
Bristol Myers CVR Dealt Blow as Key Drug Faces Approval Hurdle
(Bloomberg) -- Bristol-Myers Squibb Co.’s announcement that U.S. regulators weren’t able to meet a targeted decision deadline for a lymphoma cell therapy dealt a blow to investors as hopes for a deal sweetener for its purchase of Celgene Corp. faced more uncertainty after a rocky month.
The Food and Drug Administration said it was not able to meet a Nov. 16 target date because it was was unable to conduct an inspection of a third-party manufacturing facility in Texas, the New York-based company said in a statement. The agency is deferring action on the application until the inspection can be completed and did not provide a new anticipated action date.
The decision affects Bristol Myers’ all-or-nothing $9-a-share deal payment that requires approval by year-end of its CAR T cell therapy liso-cel as a treatment for adults with relapsed or refractory large B-cell lymphoma. The contingent value right, or CVR, crashed as much as 70% as some investors threw in the towel, though losses were roughly 30% at 4:35 p.m. in New York.
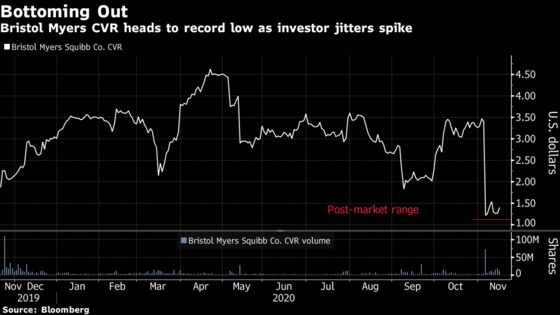
The FDA’s inability to meet the goal isn’t all that surprising given Bristol Myers said earlier this month that regulators hadn’t scheduled a required inspection of the Texas facility.
While the delay is a setback for the company and will only cause more investor jitters, Mizuho analyst Salim Syed had said Bristol Myers could still meet the year-end deadline if the agency was able to quickly visit the Texas plant. He said the FDA would need only about three weeks after an inspection to “finish up all the paperwork and get a drug to the finish line.”
In order to receive the full value of the CVR, investors also need Bristol Myers to gain FDA approval of its Bluebird Bio Inc.-partnered drug ide-cel by March 31. The therapy should get a decision from U.S. regulators by March 27.
©2020 Bloomberg L.P.