Europe’s Drug Regulator Is Backing Away From the U.K.
Europe’s Drug Regulator Is Backing Away From the U.K.
(Bloomberg) -- Europe’s medicines watchdog is bracing to make do without one of its most trusted partners.
The European Medicines Agency stopped assigning work to the U.K.’s pharmaceutical regulator earlier this year, unsure whether the two organizations will need to stop collaborating after Britain leaves the European Union in March. Preparing for a potential split, the EU body has also handed over responsibility for about 370 drugs that were overseen by the British authority to other countries.
The EMA already stands to lose hundreds of staff in its move to Amsterdam from London, and looming lack of access to British scientists adds to the pressure. Prime Minister Theresa May brought back a divorce deal from Brussels last month that will be put to U.K. lawmakers to accept or reject at a vote scheduled for Dec. 11. Approval looks unlikely, opening up the possibility the country will crash out of the bloc without an agreement.
“We have to work toward the worst-case scenario,” EMA Executive Director Guido Rasi said in an interview in London. That would mean U.K. experts “are not part of the system. They cannot be given any role. We cannot share official data. We have to unplug.”
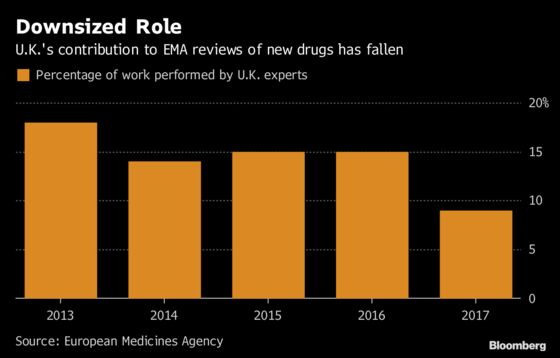
Europe’s equivalent of the U.S. Food and Drug Administration appoints teams from different nations to assess new products, and the U.K. has typically shouldered at least 15 percent of the work. The prospect of the two regulators diverging has sparked concerns about a slower process that might hinder U.K. patients’ access to new drugs.

“A significant diversion of the U.K. and EU frameworks in the future will translate into a duplication of regulatory efforts, with extended time frames and cost increases,” said Liz Cohen, a life-sciences lawyer at Bristows.
The U.K.’s planned divorce has also forced the EMA into a difficult relocation. The agency is seeking to limit losses among its 900 staff to about 24 percent, said Rasi, a physician and former head of Italy’s drug regulator. While that would be better than the earlier estimate of 30 percent, the impending shift has slowed training and technology improvements, he said.
It’s still unclear what kind of ties the U.K. regulator, called the Medicines and Healthcare Products Regulatory Agency, will have with the EMA, which oversees the safety of medicines in a market of more than 500 million people, although May’s draft agreement included potential cooperation.
Even if there is regulatory harmony, the European agency probably won’t be as reliant on Britain as it was in the past.
Having one dominant national regulator that shoulders more work than others “isn’t the wisest approach,” Rasi said. It’s a “big mistake culturally, scientifically, practically and organizationally.”
The EMA has been moving to spread the work around pre-Brexit. The number of countries handling the reviews of new medicines climbed to 22 last year from 16 in 2010, according to the EMA. Sweden, the Netherlands, Germany, Austria and Spain are among other major contributors.
British teams carried out just 9 percent of the drug assessments last year in the wake of the 2016 vote to leave the EU, half the share they had four years earlier, according to the EMA. The responsibilities for their existing work will be fully shifted to other countries at the end of March.
Regardless of the outcome, replacing the British regulator won’t be easy, according to Hein Van den Bos, a partner at law firm Hogan Lovells in Amsterdam. Authorities in the Netherlands and Germany are among those poised to pick up significant parts of the U.K. work, he said.
“That would mean of course that the large and important work done by the MHRA will have to be done by others, and that is a loss,” he said.
To contact the reporters on this story: James Paton in London at jpaton4@bloomberg.net;Suzi Ring in London at sring5@bloomberg.net
To contact the editors responsible for this story: Eric Pfanner at epfanner1@bloomberg.net, John Lauerman, Marthe Fourcade
©2018 Bloomberg L.P.