Biotech That Doubled on Covid-19 Frenzy Readies New Flu Vaccine
Biotech That Doubled on Covid-19 Frenzy Readies New Flu Vaccine
(Bloomberg) -- Novavax Inc. may be in for more wild swings as it prepares to report results at the end of this month for an experimental flu vaccine for the elderly.
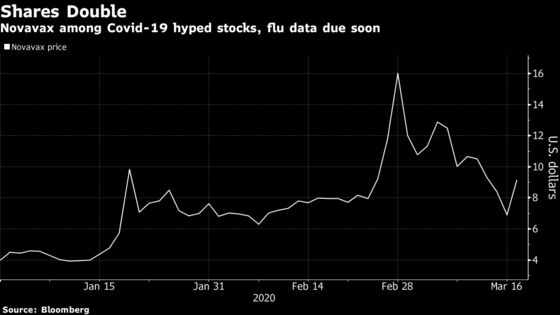
The company’s shares have more than doubled this year amid speculation that it could come up with a preventative treatment for the coronavirus that has infected at least 183,579 people and killed more than 7,400 worldwide. But with Novavax still testing that in animals, investors are looking more closely at its flu vaccine results as a more probable catalyst in the near term.
The Centers for Disease Control and Prevention estimates that influenza in the U.S. led to as many as 55,000 deaths in the current flu season and a half-million hospitalizations. NanoFlu, the biotech’s vaccine, is different than the leading flu vaccines that are produced using eggs. Instead, it uses nanoparticles to help harness immune cells, which may offer broader protection as the seasonal flu virus mutates.
The vaccine looked to spur on better antibody responses than Sanofi’s Fluzone High-Dose in prior results from a mid-stage study. Data expected by the end of this month could send shares soaring, or put Novavax closer to penny stock territory. Options expiring April 3 indicate the stock could move by about 51% in the wake of the release.
Bears may have their doubts after last year’s late-stage failure of another Novavax vaccine, ResVax, which targeted respiratory syncytial virus, or RSV, a common infection in children that can lead to hospitalization. Bets against the Maryland-based company were 20% of the available float, amounting to about $43 million, according to data from financial analytics firm S3 Partners.
Two of Novavax’s more bullish backers, B Riley FBR analyst Mayank Mamtani and Oppenheimer’s Kevin Degeeter remain optimistic. Degeeter recommended investors add to positions before the results while Mamtani saw “minimal risk” ahead of the data. Cantor Fitzgerald’s Charles Duncan upgraded shares to overweight from neutral after the close of trading Monday and raised his price target to $16 from $6.
The successful mid-stage study boosts Novavax’s chances of succeeding in upcoming data, especially as the head-to-head study compares NanoFlu to quadrivalent Fluzone, setting the bar lower than the high-dose version, Duncan said. He estimates Novavax’s U.S. unadjusted sales could exceed $600 million by 2035. The stock rose more than 30% on Tuesday after the upgrade.
Still, if Novavax’s flu vaccine works and manages to get regulatory approval, it will face some daunting competitors including Roche Holding AG, the maker of Tamiflu, as well as GlaxoSmithKline Plc and Sanofi. Last year, Citigroup Inc. said rather than competing with large drugmakers, the small-cap could draw big pharma’s eye as a takeover target.
©2020 Bloomberg L.P.