Biotech Stocks Are Enjoying Their Best-Ever Start to the Year
Biotech Stocks Are Enjoying Their Best-Ever Start to the Year
(Bloomberg) -- A string of upbeat news around this week’s JPMorgan Healthcare Conference has helped propel biotechnology stocks to their best-ever start to the year.
The Nasdaq Biotechnology Index, a bellwether of investor sentiment, has seen a 13 percent jump in 2019 after Bristol-Myers Squibb Co. kicked off the year with a record takeout of Celgene Corp. The nine-day winning streak is the longest in five years and the best start to a year since the index was created in 1993. The broader S&P 500 is up 3.6 percent year to date, while the S&P 500 Health Care Index is up just 1.6 percent.
“It really is amazing how investor sentiment can turn on a dime and how it takes one piece of big news to do that,” Brad Loncar, chief executive of Loncar Investments, said in an interview at the conference in San Francisco. But after a tumultuous fourth quarter, biotech investors still need to see more deals and easing macroeconomic headwinds “to get us back on the right track,” he said.
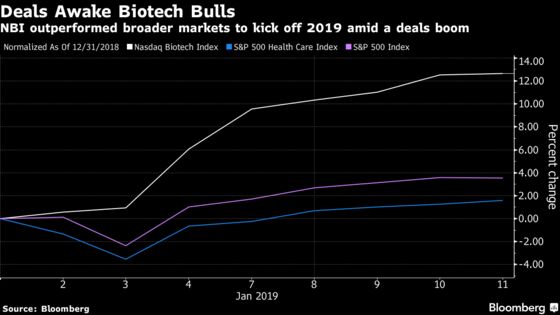
Highlights around the year’s largest health-care conference included a pair of multibillion dollar takeovers, bullish commentary from management teams and strong data on an experimental treatment for postpartum depression. Analysts have predicted an uptick in takeouts of both drugmakers and medical device companies in 2019.
While health stocks tend to be thought of as a safe haven in times of volatility, the impact of the U.S. trade war with China and growing debate around drug pricing has contributed to uncertainty over valuations.
“We got a sense that the angst that had defined the Street in late 2018 has begun to shed with the focus clearly on new ideas and less concentration on the broader market,” Jefferies trading specialist Jared Holz said after the conference.
The ongoing government shutdown may pose a risk to how long that optimism will last. Neurocrine Biosciences Inc. Chief Executive Officer Kevin Gorman said that the backlog of products awaiting review at the U.S. Food and Drug Administration “would start to become significant for the industry” if the impasse drags on for another week or more.
Neurocrine still plans to file a new drug application with the FDA next quarter. “I don’t think anyone imagines the shutdown going to that,” Gorman said in an interview.
--With assistance from Sebastian Pellejero and Jeremy R. Cooke.
To contact the reporter on this story: Bailey Lipschultz in New York at blipschultz@bloomberg.net
To contact the editors responsible for this story: Catherine Larkin at clarkin4@bloomberg.net, Sebastian Silva
©2019 Bloomberg L.P.