Biotech Stock’s 320% Rally Faces Test With Drug Data Looming
Biotech Stock’s 320% Rally Faces Test With Drug Data Looming
(Bloomberg) -- BioXcel Therapeutics Inc. has been one of Wall Street’s hottest biotech bets as analysts set sky-high price targets and pitched new drugs for brain diseases and tough-to-treat cancers -- all before seeing any late-stage trial results.
With a string of data due in the coming weeks, investors will finally get to see whether the company can live up to its more than 320% rally over the past 12 months. BioXcel is set to report results from its first two pivotal studies this month and also will release data from an earlier trial of the same drug, BXCL501.The drug, a film of dexmedetomidine taken under the tongue, is being tested as a treatment for agitation in patients with neurological disorders.
“These are the two, three catalysts that are basically going to change the profile of the company,” Guggenheim analyst Yatin Suneja said in an interview. BioXcel hasn’t shown results from a pivotal study, “so that de-risking needs to happen,” he said.
The stock’s meteoric rise has increased Wall Street’s focus on the data. If BioXcel can notch wins in the pivotal studies in schizophrenia and bipolar patients, shares could rally another 40%, according to Suneja. However, disappointing results would send the stock spiraling and wipe out close to two-thirds of its value, he said.
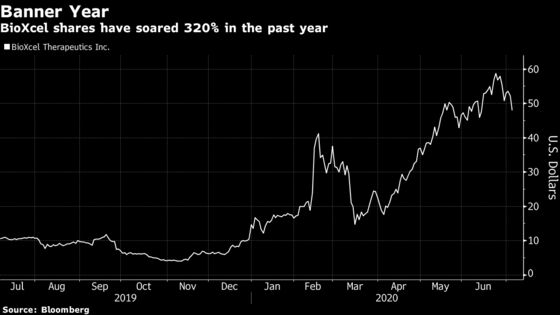
BioXcel’s advancing product pipeline has drawn some big calls across Wall Street. In February, SunTrust more than quintupled its price target to a Street-high $150 from $24, sending shares soaring. The average 12-month price target held by sell-side firms is $85, implying a 77% gain from Monday’s close.
Positive data from the pair of trials -- dubbed “Serenity I” and “Serenity II” -- could “unequivocally position” BioXcel as a key player in the neuropsychiatry and neurology fields, H.C. Wainwright analyst Raghuram Selvaraju said in a June note.
Selvaraju likened the turning point to the success of peers Axsome Therapeutics Inc. and Karuna Therapeutics Inc., which surged at the end of last year after positive results. Selvaraju said highly statistically significant results from the “Serenity” trials may enable BioXcel to trade at a valuation comparable to Axsome, which is worth $3 billion, and Karuna, which carries a $2.6 billion market value. BioXcel’s market value was $971 million as of Monday.
Guggenheim’s Suneja is optimistic after encouraging results from an early-stage trial of BXCL501 in schizophrenic patients. Suneja sees more risk for the bipolar study, though he sees the trials as likely to succeed.
BioXcel’s rise has also drawn skeptics. Bears have piled in with 11% of available shares currently sold short, according to data compiled by S3 Partners.
Developing drugs for disorders tied to the brain and the central nervous system has historically been a risky bet for investors. In December, Sage Therapeutics Inc. plummeted 60% after its experimental drug for major depressive disorder failed in a key study.
Even if BXCL501 wins U.S. approval, the potential uptake of the drug will still depend in part on pricing and getting on hospital formularies, according to Suneja.
“Investors may have concerns about the marketability and uptake of ‘501” following the “commercial failure” of Alexza Pharmaceuticals Inc.’s Adasuve, Suneja wrote in a June note. The drug was approved by the FDA in 2012 for agitation associated with schizophrenia or bipolar disorder, but less than three years later Alexza said Adasuve sales were lower than projected.
SunTrust analyst Robyn Karnauskas said she is just as focused on results from the earlier-stage “Tranquility” study testing BXCL501 as an acute treatment of agitation associated with geriatric dementia. She expects the results after the “Serenity” trials. BioXcel has said it will report the data mid-year.
“The market for dementia we think is much more tangible for investors to understand,” Karnauskas said in an interview. “It’s given in a different setting, not a hospital setting, so it’s easy to understand how you would often need a drug like this.”
Guggenheim’s Suneja said BioXcel could gain another 20% to 30% if the dementia trial is positive.
More catalysts are on the horizon. Initial data from a trial evaluating BXCL701 in prostate cancer are expected toward the end of the year. The company has also said it will provide an update on BXCL501 as a treatment for patients with symptoms of opioid withdrawal in the first quarter of 2021.
©2020 Bloomberg L.P.