Biotech Braces for Busy Summer as 2019 M&A Volume Heats Up
Biotech Braces for Busy Summer as 2019 M&A Volume Heats Up
(Bloomberg) -- Investors may be digesting a deal from Bristol-Myers Squibb Co. for Celgene Corp. and getting their teeth into AbbVie Inc.’s bid for Allergan Plc, but there are plenty of other large-cap biotechs and pharma still hungry for action.
“Do not sleep on the summer,” Jefferies trading specialist Jared Holz cautioned clients in a note. “Conversations with several banking contacts suggest mergers will not slow down in what are normally seasonally slow months.”
Who needs deals? The usual suspects -- large-cap drugmakers with patent cliffs and dwindling pipelines. With the top 20 drugmakers generating over $150 billion a year in free cash flow and facing slowing growth, “there’s high interest from big pharma and big biotech in making acquisitions,” Andy Acker, a lifesciences portfolio manager for Janus Henderson, said in a phone interview.
With regards to whether those deals will be mega-mergers or smaller bolt-ons, “anything is possible,” Holz said via phone. Although there aren’t as many targets left as there were six months ago, larger-scale deals are just as likely as smaller ones, he said.
He predicts the biggest catalyst for the second half may be a deal that closes before any political changes ahead of the 2020 election. “You want to beat the fundamental shift,” he said.
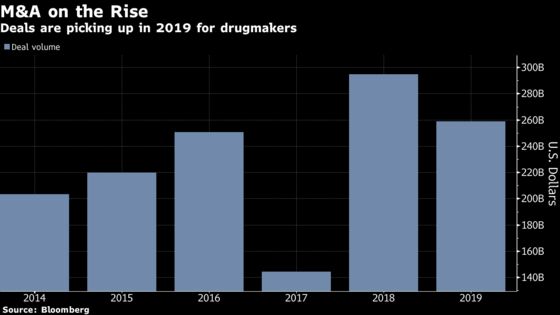
While the number of drug sector deals in 2019 to-date are on pace to match 2018’s total, the volume has exceeded last year’s midpoint thanks to mega-cap deals, according to Bloomberg data.
Potential Targets
Vertex Pharmaceuticals Inc., Incyte Corp. and Biogen Inc. are seen among the most likely targets for a large deal. Jefferies’ Holz and Cantor Fitzgerald analyst Alethia Young both called Biogen a “wildcard,” as the company could either be a buyer or seller. Other potential acquirers include Eli Lilly & Co., Pfizer Inc., Merck & Co., Sanofi, Novartis AG and Johnson & Johnson, as well as biotechs Gilead Sciences Inc. and Amgen Inc.
While large caps launch the bulk of new drugs, more than half of the new medicines approved are developed from small and mid-cap company pipelines, according to Acker. This makes biotech catalysts a key focus for investors looking to own a stock before it gets bought.
Still, 90% of new medicines in development never make it to market, Acker cautions, and for those drugs that do win regulatory approval they will still face a commercial risk, especially if they don’t get bought.
Jefferies analyst Michael Yee found that the shares of half of all biotechs that didn’t get bought underperformed in the months that followed a new drug launch.
Highlights from biotech calendars for the second half:
- Amgen: A court decision or settlement on Amgen’s dispute with Sandoz over patents for its top-selling arthritis drug Enbrel. Analysts predict Amgen shares could move as much as 10%.
- Amarin Corp.: A Food and Drug Administration decision on Sept. 28 to update the label for the heart pill Vascepa will be “the gatekeeper for M&A,” Roth analyst Yasmeen Rahimi said.
- AnaptysBio Inc: Investors are waiting for results for etokimab in atopic dermatitis; shares may rise up to 60% on positive results, JPMorgan analyst Anupam Rama predicted last month.
- Enanta Pharmaceuticals Inc: Initial results from a NASH study expected in the third quarter.
- Gilead Sciences: Results evaluating NASH combinations expected to readout in the fourth quarter and may determine the biotech’s direction in the liver disease after a recent failure.
- GlycoMimetics Inc: Results from a study of Pfizer Inc-partnered rivipansel for sickle-cell-related symptoms. Pfizer called out the drug as a potential blockbuster
- Mirati Therapeutics Inc: The stock has been breaking records since results from an Amgen study, viewed as a sign that Mirati’s cancer drug may work even better when the small-cap reveals clinical data.
- Sage Therapeutics Inc: Results in bipolar disorder patients with major depression will be closely watched by investors.
- Reata Pharmaceuticals Inc: Data from a study of bardoxolone in patients with Alport syndrome, and another study of omaveloxolone in Friedreich’s ataxia.
- WaVe Life Sciences Ltd: Two readouts expected for Wave, one in Duchenne muscular dystrophy and another in Huntington’s disease.
- Vertex: Approval for Vertex’s triple combination pill for cystic fibrosis expected this year, although that could slip into 2020, Young said.
--With assistance from Karen Lin.
To contact the reporter on this story: Cristin Flanagan in New York at cflanagan1@bloomberg.net
To contact the editors responsible for this story: Catherine Larkin at clarkin4@bloomberg.net, Morwenna Coniam, Lisa Wolfson
©2019 Bloomberg L.P.