Biogen’s Good Quarter Delays Drugmaker’s Future Reckoning
Biogen’s Good Quarter Delays Drugmaker’s Future Reckoning
(Bloomberg) -- Biogen Inc. raised its financial forecast for the year after topping second-quarter sales and earnings estimates, but the good news masks a worrying longer-term outlook for the biotechnology giant.
Part of the reason why the company was able to boost guidance Tuesday was due to lower spending on research. Biogen stopped a late-stage trial of an Alzheimer’s drug in March, a failure that has sent its shares into a monthslong slump. That drug was the most watched of its experimental therapies.
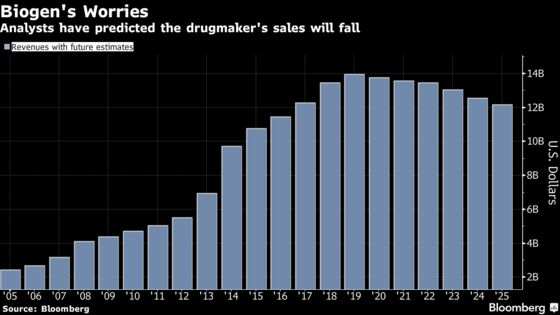
In the pharmaceutical industry, spending on R&D is an expensive necessity. While a drug’s failure may temporarily improve the bottom line thanks to lower costs, it’s disastrous for long-term growth. In the past 12 months, Biogen has lost more than $30 billion of market value, making it among the lowest-valued companies in biopharma.
The shares rose 5.3% to $244.86 at 9:49 a.m. in New York.
The company said it expects revenue this year to be between $14 billion and $14.2 billion, up from a January forecast of $13.6 billion to $13.8 billion. Its adjusted earnings-per-share for the quarter were $9.15, while analysts had anticipated $7.53.
It now stands to be seen which of the other treatments in Biogen’s research pipeline might replace sales from its aging multiple sclerosis franchise, which remains the backbone of the Cambridge, Massachusetts-based company’s sales.
Biogen Chief Executive Officer Michel Vounatsos said on a call with analysts that the company was “refining” its R&D strategy and would look to make some changes to its pipeline of drugs. Biogen now sees ophthalmology as a core franchise, and sees immunology as an emerging research area. It will also increase its focus on neuromuscular disorders.
For now, the company’s key growth driver is Spinraza, a therapy that treats the deadly childhood disease spinal muscular atrophy. While Spinraza is highly effective, a recently approved competitor from Novartis AG appears to cure the disease.
Biogen’s drug will serve as either a case study or cautionary tale of how legacy drugs will fare in the age of genetic cures for dire diseases. Spinraza’s second-quarter sales were $488 million, down from a quarter prior, and below the $530 million projected by analysts. Biogen said that Novartis’s gene therapy, which was approved in May, had yet to have a meaningful impact on its drug’s sales.
“Results were overall good and we like the general execution,” Jefferies analyst Michael Yee said in a note to clients. “However, investors aren’t looking to invest in Biogen for quarters and buybacks -- but for an improving financial outlook and better visibility on 1-5 year growth trajectory.”
--With assistance from Karen Lin.
To contact the reporter on this story: Rebecca Spalding in Boston at rspalding@bloomberg.net
To contact the editors responsible for this story: Drew Armstrong at darmstrong17@bloomberg.net, Timothy Annett, Mark Schoifet
©2019 Bloomberg L.P.