Biogen’s Costly, Unproven Drug Feared as Health Budget Buster
Biogen’s Costly, Unproven Drug Feared as Health Budget Buster
(Bloomberg) -- The approval of Biogen Inc.’s $56,000-a-year Alzheimer’s therapy creates an unprecedented challenge for the U.S. health system: a drug that many patients may get at a high price even though it may not slow their cognitive decline.
The U.S. has coped with costly, effective medicines for large populations, like those for hepatitis C, HIV and heart disease. And it has delivered therapies of less certain benefit for relatively rare diseases, such as some cancers or Duchenne muscular dystrophy.
Those drugs mainly provide solid, proven gains for patients. With Monday’s Food and Drug Administration clearance of Biogen and Eisai Co.’s Aduhelm, the health system is taking on a treatment that as many as 1 million people might qualify at a total annual cost of as much $50 billion. Despite the large burden that represents, there is little certainty about whether patients will be better off.
Biogen executives defended the price for its potential blockbuster on a call Tuesday morning and said they expect the number of patients getting it will ramp up gradually.
“We believe that the price is substantiated by the value it is expected to bring to patients, caregivers, and society,” Biogen Chief Executive Officer Michel Vounatsos said.
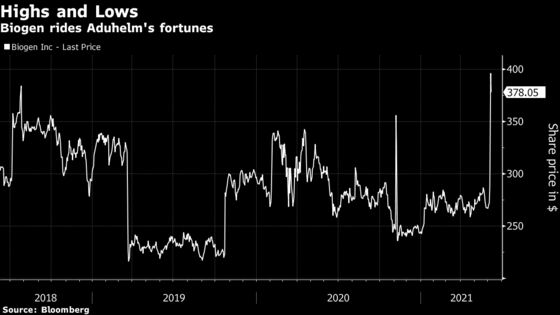
Biogen shares fell as much as 3.7% at 10:19 a.m. Tuesday in New York after reaching a record high of almost $396 Monday.
The company said it expects about 80% of patients to be covered by Medicare, the federal insurance program for people 65 and older. A spokesman for the Centers for Medicare and Medicaid Services who asked not to be named said the agency was reviewing the FDA’s decision and will have more information soon.
Averting Spending
The lifetime cost of caring for someone with Alzheimer’s is $500,000, and dementia care’s annual burden on the U.S. health-care system is about $600 billion, according to Biogen. A drug that keeps people healthy could avert expensive in-home or nursing-home care.
For a long time, “people have worried about how we would absorb the spending for an effective Alzheimer’s drug,” said Rachel Sachs, a health law professor at Washington University in St. Louis. “Part of the answer was always that an effective Alzheimer’s drug would help avert other health-care spending.”
But Aduhelm was approved because it removes abnormal protein clumps, called amyloid plaques, from the brain -- not because of dramatic relief of Alzheimer’s symptoms. The FDA said the effect on amyloid is “reasonably likely to predict a clinical benefit” and has ordered further study to confirm that.
It will likely be years before a verdict, and in the meantime, the drug threatens to simply add to an already-expensive problem.
‘Enormous’ Impact
The situation has reignited America’s simmering debate about drug prices and access to medical care. Wall Street analysts noted that the price tag exceeded expectations -- one analyst called it “daring” on Biogen’s part -- and could trigger backlash, just as costly but effective hepatitis C cures did.
“The budget impact here, just like for other therapeutic breakthroughs in the past, is going to be enormous, and it’s going to hit very quickly,” said Rena Conti, a drug-pricing expert at Boston University’s Questrom School of Business.
While Biogen and Eisai study the drug’s efficacy, they can market it and physicians can administer it via monthly infusions. But the question of who gets it at what cost is left for doctors, insurers, government health programs and manufacturers to sort out.
Aduhelm was tested in patients with early signs of Alzheimer’s and likely presence of amyloid plaques. Biogen estimates that 1 to 2 million Americans fit that description, but the FDA wrote a broad label indicating it for “the treatment of Alzheimer’s disease.”
At the announced price, treating a million patients would cost more than $50 billion a year for the drug alone, noted the Institute for Clinical and Economic Review, a nonprofit that evaluates drug costs and benefits.
While that doesn’t take into account discounts that drugmakers often give to large purchasers, it could still dramatically increase Medicare’s costs for drugs delivered in outpatient settings like physicians’ offices and infusion centers. Total spending for these Part B category drugs, under which Aduhelm falls, was $37 billion in 2019, according to Kaiser Family Foundation research.
Peak Sales
The Part B payment mechanism creates incentives for providers to prescribe the drugs, because they earn a percentage fee on the sale of the drug.
“There are financial incentives inherent to our system that are going to push for the utilization of this drug,” Boston University’s Conti said.
Still, some insurers predict far fewer than a million patients will get the drug to start.
“You’re looking at probably tens of thousands of patients being treated in the first year or two, not millions for sure, probably not even hundreds of thousands,” said Steve Miller, chief clinical officer at insurer Cigna Corp. in an interview.
Biogen told investors in a presentation that it expects “modest revenue” in 2021 as Aduhelm sales ramp up, eventually reaching a “multi-billion dollar U.S. sales opportunity over the next several years.” The company kept its annual forecast intact. Analysts at Barclays estimate 2023 Aduhelm sales of $4 billion, with potential to reach $11 billion at peak.
But beyond the cost of the drug itself, there will likely be needed cognitive screenings and brain scans to monitor for side effects. Medicare currently doesn’t cover the PET scans used to confirm amyloid in the brain.
Doctors may begin administering it within weeks and submitting bills for reimbursement that will be subject to evaluation from Medicare and private insurers. Medicare can issue a national coverage determination on how to pay for the drug.
Private Medicare plans, commercial health plans and state Medicaid programs may make their own decisions, but they often take their lead from the agency.
Humana Inc., which covers millions of Medicare members in private plans, will look to CMS “for guidance to ensure that there is consistent access to this therapy in the Medicare program,” a company spokesman said in an email.
Biogen and Cigna plan to enter into a “value-based contract to ensure that there is a streamlined path to access treatment” for patients like those in the trial, the companies said in a statement.
Uncertain Reimbursement
For any individual patient, though, it may be months before coverage determinations are clear, depending on that person’s source of insurance.
“It’s just a lot of uncertainty about what’s going to be reimbursed and when it’s going to be reimbursed,” Conti said.
Patients on Medicare are responsible for 20% of costs, with no out-of-pocket limits, unless they have supplemental coverage from private insurers or Medicaid. Who will cover those costs, and how, while the disease may continue to ravage patients, presents a dilemma that will play out over the coming years.
“A marginally effective or an ineffective Alzheimer’s drug,” said Washington University’s Sachs, “is potentially a real crisis for drug pricing and spending.”
©2021 Bloomberg L.P.