Biogen Downgraded by Baird Analyst Over Move to Salvage Alzheimer’s Drug
Biogen Gets New Bear as Baird Sees Alzheimer’s Deja Vu Risk
(Bloomberg) -- Biogen Inc. suffered another bearish sell-side call as Robert W. Baird analyst Brian Skorney cut his rating and warned investors ahead of a company presentation at a medical meeting later this week.
The downgrade comes just over a month after Biogen’s shocking move to salvage its Alzheimer’s treatment, aducanumab, sparked its best one day session in 20 years. The stock’s subsequent jump back above Skorney’s 12-month price target drew him to become the fourth analyst tracked by Bloomberg to rate it a sell, the most since April 2011.
The stock’s set-up into a Thursday presentation at the Clinical Trials on Alzheimer’s Disease meeting in San Diego “looks like Deja Vu,” Skorney wrote in a note to clients. “We expect pretty broad disappointment” as investors battle “FOMO into a regulatory decision” on the drug that is likely a year away, he wrote.
“It is underappreciated that CTAD (Clinical Trials on Alzheimer’s Disease) could serve as a meaningful negative catalyst,” Skorney said, “as we think the hype around Alzheimer’s Disease will descend into broader critique of the full data set and Biogen’s decision to file on it.” Skorney’s $250 price target is among the lowest on Wall Street, data tracked by Bloomberg show.
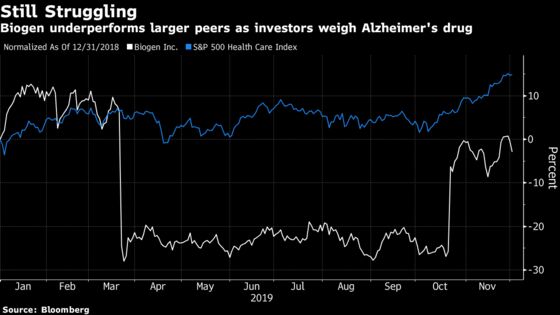
Biogen shares fell 1.9% at 9:39 a.m. in New York on Monday. The Cambridge, Massachusetts-based company ended November flat on the year, underperforming a 15% gain for the S&P 500 Health Care Index and making it one of the group’s worst performers. American depositary receipts for Eisai Co., Biogen’s partner on aducanumab, fell as much as 2.1% in U.S. trading.
Biogen crashed 29% back in March when it said the experimental Alzheimer’s drug wasn’t likely to benefit patients. The disease -- which has no currently approved treatments -- has been a graveyard for drug developers, with numerous companies failing. A potential drug approval would likely result in billions in sales given the millions of untreated patients.
While many on Wall Street rushed back to the company on the plans to march forward with the drug, Skorney wrote that there are “so many reasons we think aducanumab is getting rejected, our word count restrictions don’t allow us to put them all here.”
He added that “the FDA standard of approval is substantial evidence of efficacy and the cumulative data for aducanumab falls really far short of this standard to anyone but the most ardent GOOP subscribers.”
Skorney last recommended shares to clients in April 2018, when he held an outperform rating for less than three months. That’s the only stretch he held a buy-equivalent rating since he first downgraded shares to neutral in July 2015.
To contact the reporter on this story: Bailey Lipschultz in New York at blipschultz@bloomberg.net
To contact the editors responsible for this story: Catherine Larkin at clarkin4@bloomberg.net, Scott Schnipper, Bailey Lipschultz
©2019 Bloomberg L.P.