AstraZeneca Stock Vulnerable If Vaccine Falls Short of High Bar
AstraZeneca Stock Vulnerable If Vaccine Falls Short of High Bar
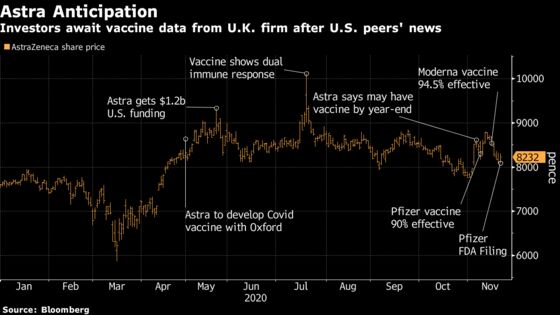
(Bloomberg) -- AstraZeneca Plc has some tough acts to follow when it rolls out final-stage test results for its Covid-19 vaccine candidate, and the U.K. drugmaker’s stock price may take a hit if it falls too far short of its competitors.
Clinical-trial results this month for two other inoculations, one from Pfizer Inc. and BioNTech SE and the other from Moderna Inc., showed those shots to be about 95% effective in preventing symptomatic infection. The University of Oxford said Thursday that key findings from the last phase of tests in its trial with AstraZeneca are expected in the coming weeks.
AstraZeneca will struggle to come close to those other candidates, according to investors and analysts interviewed by Bloomberg News, and its stock price may take a hit if it falls too far short. Daniel Mahony, a health-care fund manager with Polar Capital LLP in London, said he is looking for around 90% efficacy from AstraZeneca.
“Disappointing would be anything less than 80%,” he said. “If the data are disappointing there must be something in the share price and so the stock will be weak.”
AstraZeneca’s vaccine candidate uses a different technology to those from Pfizer and Moderna. The U.S. companies are testing vaccines that use messenger RNA, a genetic material that instructs the body to make viral proteins that in turn trigger an antibody response. AstraZeneca is using a viral vector technology that deploys an altered common cold virus to carry the genetic material of the coronavirus and produce an immune response.
“It really is best case and a very high bar,” Shore Capital analyst Tara Raveendran said of Pfizer’s readout. “It’s unlikely, based on early data that we’ve seen in terms of levels of neutralizing antibodies, that AstraZeneca would get that high.”
AstraZeneca’s shares climbed after the company said in late April it would be making an experimental coronavirus vaccine developed by Oxford and reached a peak in mid-July when early human testing showed promising results. The stock has dropped in November following the read-outs from Pfizer and Moderna.
Analysts at Barclays Plc put consensus expectations for what would be deemed a success from AstraZeneca at 70% to 90%, following conversations with investors in Europe and the U.S. While most aren’t expecting it to be as effective as Pfizer or Moderna’s vaccine candidate, they “do expect it likely works based on available clinical data and that it also is targeting the virus’s spike protein,” according to analyst Emily Field.
The U.S. Food and Drug Administration has said any shot would need to prevent disease or decrease severity in at least 50% of those vaccinated. Even so, if AstraZeneca’s data were to show 50%-60% efficacy, it will be a disappointment and hurt the shares on the day, Shore’s Raveendran said.
“I guess the problem is really more about public confidence in the vaccine,” she said. “If we have one vaccine over 90% and another vaccine at 60%, I think confidence does begin to wane a little bit, rightly or wrongly.” Efficacy closer to 70% would likely prompt a positive response, she said.
Even so, there are differences which could boost an AstraZenca vaccine’s attractiveness, even with a lower efficacy rate to those of its U.S. peers. As well as being cheaper, analysts point to its ability to scale up and less stringent cold-storage requirements.
The stock’s recent retreat may give additional room for upside on a positively viewed readout, too. “A lot of the valuation upside that was priced in the beginning has now come out,” Raveendran said.
To be sure, any effect on the share price may be limited, as AstraZeneca doesn’t intend to make a profit from the vaccine at first, and it is therefore not seen having a significant impact on the company’s earnings.
“We just don’t think that it’s going to be very impactful to their bottom line,” Field said. “Our base case expectation is that shares likely move up or down low-to-mid single digits on either a positive or negative headline, but that the vaccine news is really more of a driver of sentiment rather than an event that will result in earnings revisions.”
The wider market reaction to AstraZeneca’s data may also be more muted now that other companies have gone ahead. The Stoxx 600 Index has climbed about 6.1% since Pfizer’s update on Nov. 9 while the S&P 500 has advanced 1.8%. With two potentially viable vaccines, AstraZeneca’s project is “less important than before,” Mahony said.
While markets may have now adjusted to the expectation that there will be at least one vaccine available in 2021, investor focus is now turning to other details such as supply chains and distribution and whether the vaccines can prevent the virus from spreading.
“It would be fair to say that the market is expecting positive news on clinical data and is now beginning to look for timelines for when the vaccines will be distributed and also the takeup, given there is plenty of anti-vax commentary out there,” Ketan Patel, a fund manager at EdenTree Investment Management Ltd., said in an email.
For Mahony, who sees a minor financial impact for AstraZeneca, the key question for all the vaccines is the duration of protection. “Will people need a vaccination every year, every three years or will immunity last for years?,” he said. “This is important for us, as a society --and the associated logistics -- and also for assessing the long-term commercial opportunity there is for these companies.”
©2020 Bloomberg L.P.