Astra CEO Lands Mega-Deal, Defends Vaccine in Sydney Quarantine
Astra CEO Lands Mega-Deal, Defends Vaccine in Sydney Quarantine
(Bloomberg) -- Pascal Soriot, the head of AstraZeneca Plc, spent around two weeks in a Sydney hotel room with guards outside the door, stuck in quarantine after entering Australia.
During that time, he helped put the finishing touches on a $39 billion acquisition, while also parrying concerns over the much-anticipated Covid-19 vaccine AstraZeneca developed with the University of Oxford. That two such important developments should emerge while he was in confinement shows how crucial this moment is for the drugmaker — and for Soriot's legacy. Not even the chief executive officer of one of Britain’s most valuable companies can escape the pandemic’s grasp. But Soriot didn’t let it slow him down.
“Only Pascal Soriot would try to pull off a deal of this size while also working on a Covid-19 vaccine,” said Ketan Patel, a fund manager at EdenTree Investment Management Ltd., which holds AstraZeneca shares.
AstraZeneca announced Saturday it plans to buy rare-disease specialist Alexion Pharmaceuticals Inc. in a cash-and-stock deal to add treatments for uncommon blood and immunological disorders. The purchase of the U.S. biopharma outfit — the largest deal for AstraZeneca since it was founded in a 1999 combination of British and Swedish firms — would entrench the U.K. giant’s position among the world’s 10 biggest drugmakers. Soriot, whose quarantine ended in the early part of last week, described the acquisition on a call with reporters as “an important step in the history of the company.”

Investors’ doubts about the merits of the acquisition sent AstraZeneca shares tumbling as much as 9.2% in early trading, the biggest intraday decline since March. The stock was down 5.2% at 8:15 a.m. in London.
The transaction may be a distraction of sorts for AstraZeneca from a spate of negative commentary in recent weeks around its vaccine effort, after the company and Oxford published results from clinical trials. While the studies showed the shot is safe and effective, they also generated concerns.
Crucial facts, such as how well the vaccine works in older people — among the most at risk from the virus — couldn’t be deduced and will require more analysis. A manufacturing mistake led to two different results from two dosing regimens, creating confusion over the best quantity to use and the vaccine’s overall efficacy. Piecemeal disclosure of these and other key details revealed a puzzling lack of transparency on such a high-profile effort.
While no one disputes that creating an effective vaccine in less than a year is a huge achievement, the miscues could set back approval and deployment of the potentially life-saving shot, particularly in the U.S. In an interview during his confinement, Soriot downplayed the criticisms, blaming “armchair quarterbacks” who have blown matters out of proportion.
“People are dying, the economy is crumbling, people are losing their jobs,” said the Frenchman, who traveled to Australia to see his family after 10 months apart because of the virus. “We need to find a way to put an end to this terrible situation with a good vaccine that is safe, and that’s what we have.”
For Soriot, 61, landing in the hotseat is largely his own doing. Known for producing innovative and lucrative cancer treatments rather than vaccines, AstraZeneca wasn’t the obvious choice to partner up with Oxford when scientists there started developing the coronavirus shot. Yet never one to shy away from a challenge — or opportunity, as the Alexion purchase shows — Soriot jumped in.
His decision to join the vaccine project in April — when much of the world’s population was in lockdown — put the drugmaker at the center of a race to conquer a pathogen that has now claimed more than 1.6 million lives and wrought widespread economic damage. When early studies showed promise, the Astra-Oxford shot soon drew attention as one of the leading vaccine candidates.
By mid-June, more than one billion doses had been ordered by governments, including the U.S. and U.K. The shot, though still in trials, became attractive to swaths of the world because AstraZeneca and Oxford pledged to provide it at cost — just a few dollars a dose — until the pandemic ends.
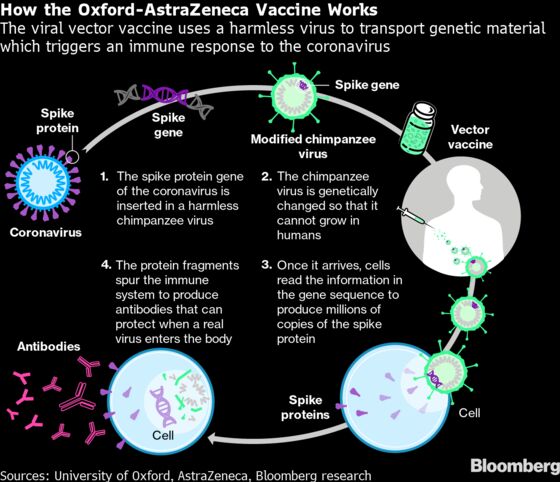
The data AstraZeneca and Oxford released last month showed that the shot works, but left doubts over how much protection it would provide. Counter-intuitively, the partners said their vaccine was 90% effective when a half-dose was given before a full-dose booster, and that two full doses showed an efficacy of 62%.
While even that lower rate exceeds the U.S. Food and Drug Administration’s 50% threshold for deeming a shot effective, it was below the 95% level achieved in vaccine trials by partners Pfizer Inc. and BioNTech SE, and by Moderna Inc. The Pfizer shot has now been approved in both Britain and the U.S.
It later emerged the lower dose in the Astra-Oxford trials was the result of a manufacturing error and was only tested in a younger group, further clouding the picture over its efficacy because the old tend to be less responsive to vaccines. While peer-reviewed data in The Lancet last week confirmed that the vaccine is effective, the questions remain.
“Everyone is now wondering about the AstraZeneca-Oxford vaccine precisely because, frankly, we haven’t got sufficient data at this point in time to be able to make a decision on whether it is a good vaccine,” said David King, the U.K.’s former chief scientific adviser.
At the start of 2020, Soriot was riding high. After seven years leading AstraZeneca, he could claim credit for pulling off one of the industry’s most surprising turnarounds and almost tripling the share price. When he joined, the patents on its most profitable drugs were nearing expiration, with few promising replacements on the horizon. He pulled the drugmaker back from the edge and transformed it into an oncology powerhouse. In April, AstraZeneca briefly became the largest company in the FTSE 100 by market value.
The mission wasn’t always straightforward. In 2014, he fought off a $117 billion takeover offer from Pfizer, making a bet he could create more shareholder value following his own pursuits than by selling the company. It eventually paid off.
Acquiring Alexion would build on Soriot’s turnaround. The company has specialized in developing drugs that selectively inhibit immune factors to fight diseases that involve the body’s protective system. Soliris, Alexion’s biggest product, with about $4 billion in 2019 sales, is a monoclonal antibody. Similar therapies have been given emergency authorization for use against Covid.
Alexion was quietly sounding out potential buyers over the last few months and several large U.S. and European drug companies considered the acquisition, according to people familiar with the matter.
As for how Soriot pulled off the deal from confinement, “there have been many late nights and early starts, but technology has been a great enabler,” he told Bloomberg, also crediting his colleagues around the world. “The pandemic has made us rethink the way we work, and things that would have been unthinkable a year ago have changed, and possibly forever.”
Soriot’s record of success may explain why investors have mostly shrugged as he diverted energy and resources into a vaccine project that doesn’t stand to make money, at least in the near term.
It was on conference calls with his team in China in February that Soriot realized that 2020 was going to look much different from what he had expected. An unknown virus was circulating in the country and his employees were clearly putting a brave face on things.
“I could see they were dealing with a crisis,” said Soriot. “I realized, wow, this is not going to be a Chinese-only issue. It’s going to be a big deal.”
Like other pharma CEOs, Soriot started donating face masks and examining the Cambridge, England-based company’s portfolio of medications for possible treatment options. Then he heard about the Oxford vaccine.
The university had gotten an early jump on creating a shot after years spent working on other vaccines, including one for Middle East Respiratory Syndrome, a deadly disease also caused by a coronavirus. By April, the scientists were ready to begin human trials for their Covid-19 shot, but had realized they needed a partner with manufacturing expertise to deploy a successful vaccine at scale.
After initial conversations with Merck & Co. of the U.S., the view at Oxford, and within the U.K. government, was that working with a British partner would be preferable, at least in part to avoid supply issues if the vaccine became successful, according to people familiar with the matter.
GlaxoSmithKline Plc, the world’s largest vaccine company by revenue, would appear the most logical choice. But when U.K. Chief Scientific Adviser Patrick Vallance called Roger Connor, president of Glaxo’s vaccines business, it became clear the London-based company had already backed other horses, said a person with knowledge of the situation who asked for anonymity describing private discussions.
Soriot had known John Bell, an Oxford professor working with the government on its vaccine program, for years. When the pair spoke in early April, they agreed over a weekend that the drugmaker would join the effort. Three weeks later, AstraZeneca and Oxford announced their plans to develop and manufacture the vaccine together.
Emily Field, a European pharmaceuticals analyst at Barclays Plc, is sanguine about AstraZeneca’s decision to participate in the project. She described it as a “win-win” from a reputational standpoint, a rarity at a time when big drugmakers are often cast as villains. If the vaccine doesn’t work out, “investors can return to focusing on their key oncology franchises,” she said. “But if it does, the company has done what it set out to do in terms of doing good for society.”
What AstraZeneca couldn’t foresee was how bumpy the journey to a successful shot would be.
In September, global trials were paused after a U.K. volunteer suffered unexplained neurological symptoms. The British study resumed less than a week later, but the stoppage stretched to almost seven weeks in the U.S. In The Lancet last week, Oxford and AstraZeneca explained that the incident related to a volunteer who developed transverse myelitis, and said it couldn’t be determined whether the condition was linked to the vaccine or not.
The extended halt in the U.S. trial will probably delay the prospects for regulatory approval there, as the FDA is unlikely to sign off on the shot based only on the studies carried out overseas, Soriot said.

So far, the U.S. trial has recruited 17,000 of the 40,000 participants it’s seeking, and won’t read out until late January or early February, Moncef Slaoui, chief scientific officer of the U.S.’s Operation Warp Speed, has said. An emergency-use authorization in the U.S. probably wouldn’t be forthcoming before late February or early March, he added.
Despite the lingering questions surrounding the vaccine’s efficacy among older people, the U.K. regulator is likely to approve the Astra-Oxford shot by year-end, with clearance by the European Medicines Agency following shortly thereafter. Britain’s regulator approved the Pfizer-BioNTech shot earlier this month and the National Health Service began what it has called the biggest immunization campaign in its history last week.
The difficulty of pairing a pharmaceutical giant with a group of university scientists has played out in the media over recent weeks. In the wake of the interim results on the vaccine, conflicting versions of the manufacturing error raised questions over how aligned partners AstraZeneca and Oxford actually are.
Mene Pangalos, AstraZeneca’s biopharmaceuticals R&D chief and a key player in the vaccine effort, initially flagged the misstep in a Reuters interview, claiming it was “serendipity.” In an interview with Bloomberg the same day, Oxford’s vaccine lead Sarah Gilbert said researchers were simply experimenting with different dosing regimes to find out what would be best tolerated while generating a strong immune response.
In the interview, Soriot pointed out that the trials outside the U.S., which have generated controversy, were started by Oxford “before we joined.’’ The U.S. study, which AstraZeneca is running, is “just like Pfizer, like Moderna, like J&J,” he added, referring to U.S. drugmaker Johnson & Johnson.
AstraZeneca plans to manufacture more than 300 million doses of the vaccine by the end of the first quarter, and at peak delivery will supply between 100 million and 200 million doses a month globally, Pam Cheng, the company’s head of operations, said at a briefing on Nov. 23. Working with more than 20 partners in over 15 countries, including Brazil and India, the company intends to produce as many as 3 billion doses next year.
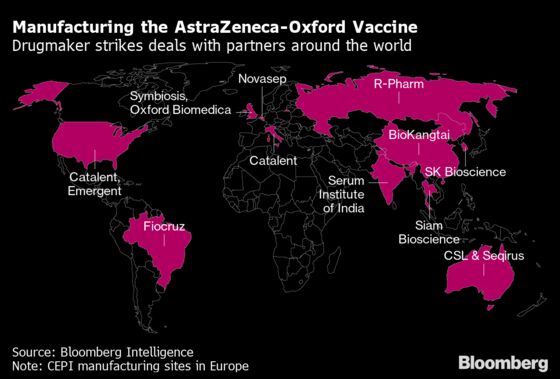
“We have not wasted a single day in setting up the supply chain,” Cheng said. “We have a potential vaccine that can be easily distributed, stored and administered.”
The Astra-Oxford shot has an advantage over those of Pfizer and Moderna, in addition to its lower cost. While the other two must be stored in a deep freeze, AstraZeneca’s can be kept at refrigerator temperatures, making it much easier to transport and use in the developing world.
Soriot said the jury is out on whether AstraZeneca will move deeper into vaccines over the longer term. With the Alexion deal to complete, management will probably have its hands full for now without other new projects, but the possibility is there.
“If it works, then that’s certainly something we will look at,” he said. “We have a few ideas of what we could do, but we just want to cross the finish line.”
©2020 Bloomberg L.P.