Annovis’s 127% Surge Showcases Alzheimer’s Drug Enthusiasm
Annovis’s 271% Surge Showcases Broad Alzheimer’s Drug Enthusiasm
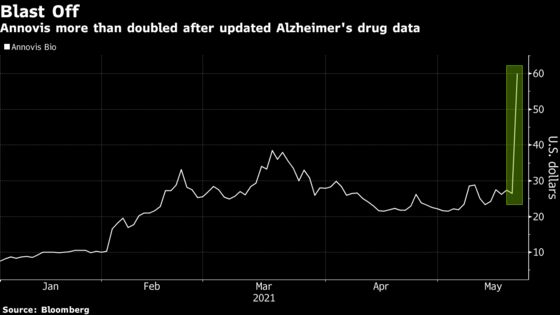
(Bloomberg) -- Annovis Bio Inc.’s 127% surge after new data from a trial of its experimental drug to combat Alzheimer’s disease and Parkinson’s disease showcased investors’ enthusiasm for stocks that are targeting the tough-to-treat brain disorders.
The fervor sent the Berwyn, Pennsylvania-based company soaring to a record high of $60 on Friday as it added around $230 million in value and millions of shares changed hands -- making it one of the day’s most actively traded stocks. The company said patients that received the drug for 25 days showed cognitive improvement as measured by an 11-part test.
The rally comes with about two weeks until a critical decision from the Food and Drug Administration over whether to approve Biogen Inc.’s experimental Alzheimer’s drug, aducanumab. The decision on the hotly debated therapy is seen as the most-closely watched event in the biotech industry this year -- and a green light may buoy shares of other firms with therapies that are less advanced.
Traders have highlighted Biogen’s Japan-based partner Eisai Co., Prothena Corp., Athira Pharma Inc., Alector Inc., and Cassava Sciences Inc. among smaller peers that could see big share reactions if Biogen’s drug wins approval. It’s worth noting that euphoria around small datasets has helped make Cassava Sciences one of the best-performing stocks in the Russell 2000 this year despite warnings from one analyst that the company is “not for the faint of heart.”
Brain-Disease Drugs Near FDA Review, With Stocks’ Fate in Play
Many of the companies that are also studying experimental drugs for Alzheimer’s saw their shares climb Friday as traders discussed the implications of Annovis Bio’s data. Cassava Sciences climbed as much as 11% at one point Friday, while Anavex Life Sciences Corp. gained as much as 6.7% and Prothena as much as 8.7%.
©2021 Bloomberg L.P.