Failure of Alzheimer’s Disease Study Creates a New Penny Stock
Failure of Alzheimer’s Disease Study Creates a New Penny Stock
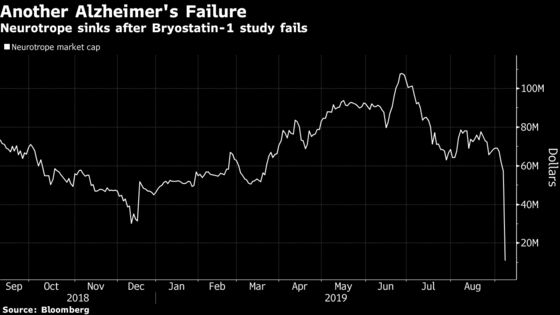
(Bloomberg) -- Neurotrope Inc. plunged as much as 82%, falling below a dollar, after a placebo worked better than the company’s experimental treatment for Alzheimer’s disease.
Bryostatin-1 showed a 1.3 point improvement on a score judging patients’ mental impairment after three months of treatment while patients getting a placebo treatment had a 2.1 point improvement. The stock fell as low as 81 cents -- a record -- on heavy volume more than 35 times the daily average.
“We are disappointed in the topline results from the confirmatory Phase 2 study,” the company’s Chief Executive Officer Charles Ryan said in a statement. “Having just received the data, we are conducting a full review to determine potential next steps.”
One of the two analysts tracked by Bloomberg who covers Neurotrope, Maxim’s Jason McCarthy, cut his rating to hold from buy, and removed his $16 price target on the company. He said he was awaiting clarity on if there will be a path forward for bryostatin.
Drug developers have been racking up failures in Alzheimer’s as they seek to find a cure for this scourge of aging. Biogen Inc.’s disappointing March results for a drug targeting beta amyloid -- a plaque that builds up in the brain of Alzheimer’s patients -- rocked the market.
Drugmakers are persisting nevertheless. Biogen, Eli Lilly & Co., Roche Holding AG as well as small-cap Cassava Sciences Inc. are developing new medicines that target a pathway called tau. Cassava reported its own early results Monday morning, focused on biomarkers of the disease. Cassava’s stock fell 9.3% at 2:25 p.m. The decline could be the result of pressure from Neurotrope’s failure, McCarthy said. Or it could be due to the small size of the study, which included just 13 patients. McCarthy rates Cassava a buy.
To contact the reporter on this story: Cristin Flanagan in New York at cflanagan1@bloomberg.net
To contact the editors responsible for this story: Catherine Larkin at clarkin4@bloomberg.net, Jim Silver, Divya Balji
©2019 Bloomberg L.P.