All-or-Nothing Bet on New Bristol Myers Drugs Faces First Test
All-or-Nothing Bet on New Bristol Myers Drugs Faces First Test
(Bloomberg) -- An upcoming U.S. regulatory decision for Bristol Myers Squibb Co.’s multiple sclerosis treatment marks the first of three key milestones that will dictate the fate of its all-or-nothing payment to former Celgene Corp. investors.
The Food and Drug Administration is slated to decide whether to approve the first medicine, ozanimod, by March 25. Many on Wall Street anticipate the drug will win approval based on solid data and the fact that regulators agreed to review the filing after it was turned down in 2018.
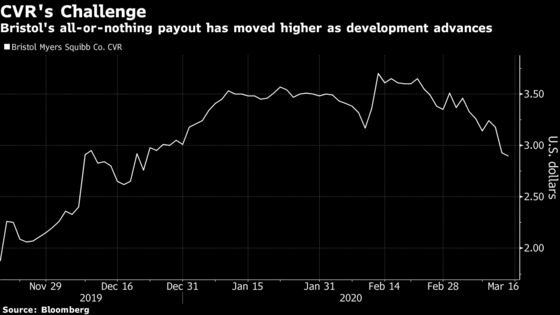
Approval would be the first step toward a potential payment that Bristol Myers used to sweeten its takeover of Celgene last year. To receive the full $9 value of the so-called contingent value right, investors also need Bristol Myers to secure FDA approval of two other former Celgene drugs by the end of March 2021. If any of the three don’t make it, the CVR is worth nothing.
“We expect approval given this is a validated mechanism and that the application was accepted after initially being rejected,” Mizuho analyst Salim Syed said in a phone interview. “The acceptance, to us, was the bigger hurdle as the data show it is effective and it looks safer than Novartis’s Gilenya.”
CVRs are tradeable securities that are occasionally included in drugmaker deals where there’s a high risk/reward for experimental treatments. Wall Street loves to bet on them, and the Bristol Myers CVR is no different. Daily trading volume this year is almost 4 million, compared to less than 2 million shares traded daily for mega-cap biotech stocks like Biogen Inc.
Read more: Celgene CVR Faces Tough Sledding to Return 300%, Mizuho Says
Syed estimates approval of ozanimod would add about a dollar to the CVR value, or roughly 33%. It has already climbed by more than 50% since it began trading in November, touching a high of $3.79 last month. Sentiment brightened after Bristol Myers said the FDA granted priority review for the second drug, lisocabtagene maraleucel, to treat a type of blood cancer. The agency assigned an Aug. 17 decision date.
A filing for the third medicine, Bluebird Bio Inc.-partnered bb2121, is expected to be submitted to regulators sometime in the first half of this year.
Matt Phipps, an analyst at William Blair, is optimistic that all three drugs will win approval but he cautioned that “weird things can happen.”
One potential risk is that the spread of coronavirus could disrupt the workings of the federal government. Syed said in a recent note that he’s “starting to get questions on what happens to the CVR if the FDA shuts down temporarily.”
Earlier this week, the FDA announced that is postponing “all non-essential meetings” through April.
To contact the reporter on this story: Bailey Lipschultz in New York at blipschultz@bloomberg.net
To contact the editors responsible for this story: Catherine Larkin at clarkin4@bloomberg.net, Kristine Owram
©2020 Bloomberg L.P.