All-or-Nothing Bet on Bristol Drugs Goes Sour as FDA Reviews Lag
All-or-Nothing Bet on Bristol Drugs Goes Sour as FDA Reviews Lag
(Bloomberg) -- The deal sweetener that Bristol Myers Squibb Co. offered as a part of its bid to buy Celgene Corp. plunged after management said U.S. regulators have not scheduled an inspection of a facility that is key for investors to get the $9 all-or-nothing payment.
The so-called contingent value right, or CVR, fell as much as 81% to a record low of 65 cents as the sweetener would become worthless if the company’s blood cancer drug lisocabtagene maraleucel isn’t approved in a timely manner.
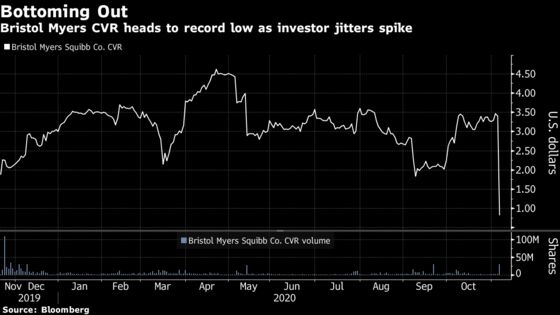
During the company’s quarterly earnings call, management said the U.S. Food and Drug Administration had not yet scheduled a required inspection of a Texas facility, which would be needed for a Nov. 16 decision date and a year-end deadline.
The derivative was worth roughly $1.88 when it started trading last November, and is also dependent on Bristol Myers, in partnership with Bluebird Bio Inc., winning approval of a second drug, idecabtagene vicleucel (bb2121) in multiple myeloma, by March 31.
CVRs are tradeable securities that are occasionally included in drugmaker deals when there’s a high risk/reward for experimental treatments. Wall Street loves to bet on them, and the Bristol Myers CVR is no different. Trading volume on Thursday topped 29 million shares in the first 20 minutes, more than five-times the average volume of the derivative and double the average for Bristol Myers stock over the past year.
“Investors were expecting, and what seemed to make most logical sense, is to inspect the plants together closely in sequence, Bothell and Texas,” wrote Mizuho analyst Salim Syed. He said it is possible for Bristol to meet the year-end deadline with the FDA only needing about three weeks after the Texas inspection to “finish up all the paperwork and get a drug to the finish line.”
©2020 Bloomberg L.P.