Aimmune Reverses Gains as Street Weighs Peanut Drug’s Potential
Aimmune Reverses Gains as Street Weighs Peanut Drug’s Potential
(Bloomberg) -- A positive recommendation from a group of advisers to the U.S. Food and Drug Administration in favor of Aimmune Therapeutics Inc.’s Palforzia indicates the peanut allergy drug will win approval, but its commercial success is less clear.
“Right now it’s probably a sell-the-news event and ultimately investors thinking about the safety” or how an anticipated risk-management program “could impact the launch,” Stifel analyst Derek Archila said by telephone.
Shares of the drug developer erased earlier gains Monday to slide 6.2%. Aimmune remains more than 5% higher since a pre-meeting report was released Sept. 11.
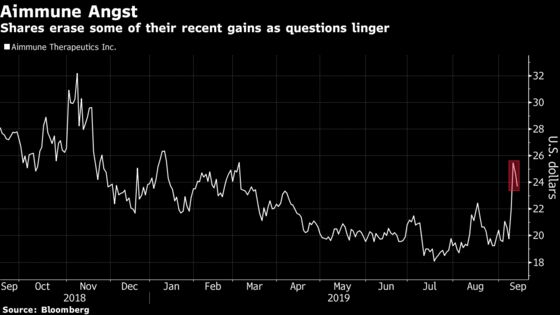
Panelists broadly supported a Risk Evaluation and Mitigation Strategy, or REMS, program that will help ensure patients and caregivers are acting safely. Recommendations for the desensitization therapy ranged from a requirement for epinephrine to dosing guidelines. Some analysts, including Archila, have said that a more onerous safety strategy could impact initial sales amid heightened expectations.
“This is not going to be for every family, and I think the panel indicated that this will be best for very highly motivated parents and patients,” Archila said. “There are also a lack of catalysts up until approval by January,” he continued.
Stifel forecasts 2024 sales of roughly $650 million, which is about half of the average Wall Street estimate for around $1.27 billion.
An FDA decision is expected by late January.
To contact the reporter on this story: Bailey Lipschultz in New York at blipschultz@bloomberg.net
To contact the editors responsible for this story: Catherine Larkin at clarkin4@bloomberg.net, Steven Fromm, Jeremy R. Cooke
©2019 Bloomberg L.P.