Pfizer's Potential Blockbuster Heart Drug Boosted by Study
Pfizer's Potential Blockbuster Heart Drug Gets Boost From Study
(Bloomberg) -- Pfizer Inc.’s pipeline may finally be delivering.
A new study shows that patients with a rare condition that can lead to heart failure lowered their risk of dying by 30 percent after being treated with Pfizer’s tafamidis. The finding could give the biggest U.S. drugmaker another potential blockbuster medicine and challenge a biotech startup marketing its own groundbreaking treatment for the same disease.
Pfizer estimates that as many as 500,000 patients worldwide have the disease known as transthyretin cardiomyopathy but that less than 1 percent have been diagnosed. Doctors also say the condition is more prevalent than what’s been reported. Pfizer said there are about 50 centers in the U.S. equipped with diagnostics to identify patients, which it expects to grow now that the data has been disclosed.
Tafamidis is expected to be the first of more than a dozen medicines the New York-based company has vowed to launch in the coming years that could generate more than $1 billion in annual sales. Some analysts project the drug will cross that threshold within five years, and possibly sooner if more patients with transthyretin cardiomyopathy are identified.
“It’s very, very exciting,” said Irina Sobol, director of the advanced heart failure fellowship program at Weill Cornell Medicine and New York-Presbyterian, who wasn’t involved in the trial. “I fully expect it would change the standard of care. It will make physicians be more excited, it will lead us all to look for patients with this disease because now there are clear paradigms for treatment.”
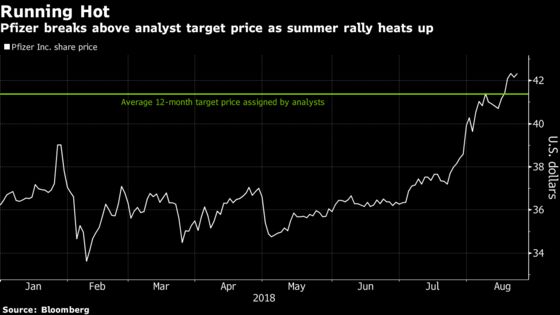
Pfizer shares, which have been trading near their highest level in more than 16 years, fell as much as 2.4 percent Monday after the company said on a conference call with analysts that it wouldn’t immediately pursue U.S. approval for the same indication that regulators recently granted to a treatment from Alnylam Pharmaceuticals Inc. Alnylam shares surged as much as 17 percent.
Transthyretin cardiomyopathy is a progressive, fatal disease caused by a protein that circulates naturally in the blood but becomes unstable and misfolds, building up in the heart and causing it to thicken and stiffen. The disease can be caused by a flawed gene, but a nonhereditary form also exists.
The data from the study, presented at the European Society of Cardiology’s annual conference in Munich and published Monday in the New England Journal of Medicine, could position Pfizer’s drug as a surprise rival to one marketed by Alnylam. The Cambridge, Massachusetts-based company’s drug, Onpattro, recently became the first medicine based on RNA interference approved in the U.S. Carrying a $450,000 annual price tag, Onpattro temporarily blocks, or silences, the messages carried by the defective genes.
Alnylam’s drug doesn’t have a proven survival benefit, and its approval was based on a study of patients who inherited the disease. The study of tafamidis, by contrast, included the nonhereditary form as well, which is more common in men over 60, according to Pfizer.
An increase in patient awareness and diagnosis of the disease could be lucrative for Pfizer. Greg Gilbert, an analyst at Deutsche Bank AG, estimates the drug will be commercially available in 2020 and sell more than $1 billion by its fifth year.
Pipeline Strength
Such fortunes would validate the company’s expectations to potentially have between 25 and 30 drug approvals by 2022, with up to half having the potential to sell more than $1 billion annually. Chief Executive Ian Read has said Pfizer is prepared to grow on the strength of its pipeline and doesn’t need a large merger or acquisition to grow.
“This is an event that Pfizer has been talking up a lot too when they lay out their pipeline,” said Vamil Divan, an analyst at Credit Suisse AG.
Pfizer surprised investors and doctors in March when it said the tafamidis study was successful because in 2012 the drug was rejected by U.S. regulators for approval to treat a different disease. Alnylam’s shares slide more than 25 percent in the three days after the announcement. (Some countries outside the U.S. approved Pfizer’s drug, now marketed abroad as Vyndaquel.)
Ionis Pharmaceuticals Inc. and its affiliate, Akcea Therapeutics Inc., are also hoping for U.S. approval of their own drug to treat the disease before year-end. The medicine, Tegsedi, is approved in Europe and operates similarly to Alnylam’s Onpattro, though both are delivered via injection. Pfizer’s drug is a chemical compound taken orally.
Once diagnosed with transthyretin cardiomyopathy, patients live between two and six years, according to researchers. Improvements in diagnostics, such as imaging and testing, have helped identify more patients, but a lack of treatments has discouraged physicians from searching for signs of the disease, Sobol said.
“Some people felt, why look for something that we cannot treat?” she said.
In a late-stage study of 441 patients diagnosed with transthyretin cardiomyopathy, those treated over 30 months with tafamidis saw their risk of dying during the trial reduced by 30 percent -- with a 32 percent reduction in hospitalizations -- when compared with patients given a placebo, according to the Pfizer-sponsored study.
“This is the first step in the right direction,” said Milind Desai, a cardiologist at the Cleveland Clinic, who wasn’t involved in the study. “It’s sort of like you see potential and the guy walks onto the mound and hits a home run in his first at-bat.”
While tafamidis showed a significant benefit to patients in the nonhereditary form, it didn’t reach statistical significance for patients with the hereditary form of the disease, according to the data.
--With assistance from Michelle Fay Cortez.
To contact the reporters on this story: Jared S. Hopkins in New York at jhopkins38@bloomberg.net;Bailey Lipschultz in New York at blipschultz@bloomberg.net
To contact the editors responsible for this story: Drew Armstrong at darmstrong17@bloomberg.net, Mark Schoifet, Timothy Annett
©2018 Bloomberg L.P.