Biogen Closes at 3-Year High Ahead of Crucial Alzheimer's Data
Biogen Awaits Crucial Alzheimer's Results as Earnings Beat
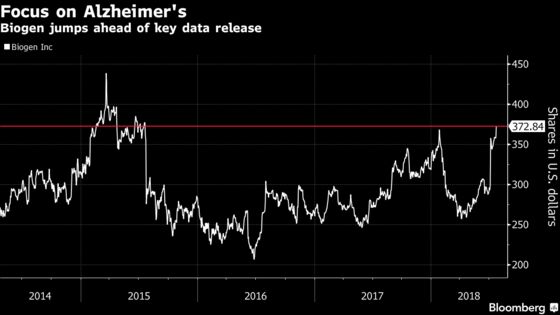
(Bloomberg) -- Biogen Inc.’s stock jumped to the highest in three years, a day ahead of the release of results for an Alzheimer’s therapy that can prove key to the drugmaker’s future.
The shares closed up 4.1 percent to $372.84 on Tuesday, the highest since July 2015, giving Biogen a market value of $78.7 billion. The stock was bolstered by strong second-quarter results, as one of the company’s newest treatment, spinal muscular atrophy drug Spinraza, handily beat Wall Street estimates.
All eyes are now on a medical meeting in Chicago Wednesday, when researchers will present results from an Alzheimer’s disease drug called BAN2401. Earlier this month, Biogen’s shares surged 20 percent in a day, after the drugmaker said the drug had slowed the course of the disease in a large trial. Details of those results, including how much patients were helped, will be crucial.
Biogen, based in Cambridge, Massachusetts, is developing the treatment with Japanese partner Eisai Co. The companies have contacted regulatory agencies to determine what steps will be necessary to get approval of the medicine, said Lynn Kramer, chief clinical officer of Eisai.
“Our intention is to do additional trials, but we want to see what the next steps will be from their perspective before we move ahead,” Kramer said of regulators.
Eisai and Biogen still have critical questions to answer in terms of the design of the next experiments with the drug. The study being presented Wednesday used a relatively rare practice that steered patients into parts of the trial that seemed to have the highest likelihood of generating a result.
“We took the three traditional regulatory endpoints and from that found the ones that moved earlier,” Kramer said. “We believe it to be the better endpoint in early disease.”
The company plans to present more traditional results to bolster the evidence it has already shown.
“We hope to validate it. This is one of the ways. But we have the support of the additional endpoints as well,” he said.
In an interview with Reuters, Kramer said the drug had also showed positive results at a lower dose, data that wasn’t included earlier this month.
On a call with investors Tuesday, Biogen Chief Medical Officer Al Sandrock said the conversations with regulators would be crucial in determining the next steps for the drug. He said it was too early to speculate on whether the company could ask the Food and Drug Administration to approve the treatment based on the data.
Alzheimer’s disease is a notoriously risky therapeutic area for drugmakers. Numerous rivals have abandoned research into the illness after high-profile trials ended in failure. Yet with no treatments on the market that can reverse the course of the disease, it remains an incredibly tempting opportunity. Biogen has a separate Alzheimer’s compound, aducanumab, with trial results from a final-stage test expected next year.
To contact the reporters on this story: Rebecca Spalding in New York at rspalding@bloomberg.net;Michelle Fay Cortez in Minneapolis at mcortez@bloomberg.net
To contact the editors responsible for this story: Drew Armstrong at darmstrong17@bloomberg.net, Timothy Annett
©2018 Bloomberg L.P.